1. Complete Activity 1.1 (Page 1).
CAUTION: This Activity needs the teacher’s assistance. It would be better if students wear suitable eyeglasses.
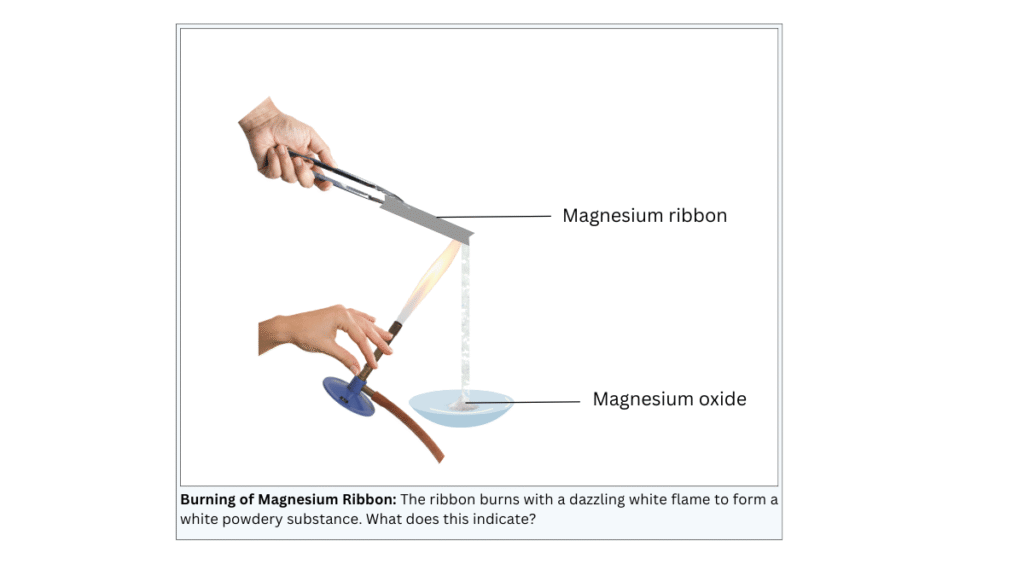
- Clean a magnesium ribbon about 3-4 cm long by rubbing it with sandpaper.
- Hold it with a pair of tongs. Burn it using a spirit lamp or burner and collect the ash so formed in a watch-glass as shown in Fig. 1.1. Burn the magnesium ribbon keeping it away as far as possible from your eyes.
- What do you observe?
Answer:
Aim: To observe what happens when the magnesium ribbon is burnt and make the necessary conclusions.
Materials Required: Magnesium ribbon about 3-4 cm long, sandpaper, tongs, Bunsen burner, watch-glass.
Procedure:
(i) The magnesium ribbon is rubbed with sandpaper.
(ii) Then it is held with the pair of tongs and burnt using the burner.
(iii) The ash is allowed to fall into the watch-glass positioned below the flame.
Observations:
- The ribbon burns with a dazzling white flame and a white powder in collected in the watch-glass.
Conclusions:
- The white powder is a new substance called magnesium oxide.
- The formation of a new substance with new properties indicates that a chemical reaction has taken place between magnesium and atmospheric oxygen. The reaction is shown below:
2Mg + O2 —> 2MgO
“CAUTION: This Activity needs the teacher’s assistance. It would be better if students wear suitable eyeglasses.
What do you observe?
Clean a magnesium ribbon about 3-4 cm long by rubbing it with sandpaper.
Hold it with a pair of tongs. Burn it using a spirit lamp or burner and collect the ash so formed in a watch-glass as shown in Fig. 1.1. Burn the magnesium ribbon keeping it away as far as possible from your eyes.” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 1.1
Solution to Activity 1.2
Solution to Activity 1.3
Solution to Activity 1.4
Solution to Activity 1.5
Solution to Activity 1.6
Solution to Activity 1.7
Solution to Activity 1.8
Solution to Activity
Solution to Activity 1.9
Solution to Activity 1.10
Solution to Activity 1.11


