7. Complete Activity 3.7 (Page 39).
- Collect samples of carbon (coal or graphite), sulphur and iodine.
- Carry out the Activities 3.1 to 3.4 and 3.6 with these non-metals and record your observations.
Answer:
Aim: To repeat the Activities 3.1 to 3.4 and 3.6 with non-metals carbon (coal or graphite), sulphur and iodine and make conclusions based on what you observe.
Materials Required: Carbon (coal or graphite), sulphur and iodine, sand paper, knife, hammer, battery, bulb, switch, electric wires, clips.
Procedure:
Repeat of Activity 3.1:
(i) Note the appearance of carbon, sulphur and iodine.
(ii) Rub the metals with sandpaper and study the appearance again. Make a note of the observations.
Repeat of Activity 3.2:
(i) Try to cut the samples of carbon, sulphur and iodine with the knife and note the observations.
Repeat of Activity 3.3:
(i) Strike the pieces of carbon, sulphur and iodine with the hammer and note the observations.
Repeat of Activity 3.4:
(i) Try to find wires of carbon, sulphur and iodine.
Repeat of Activity 3.6:
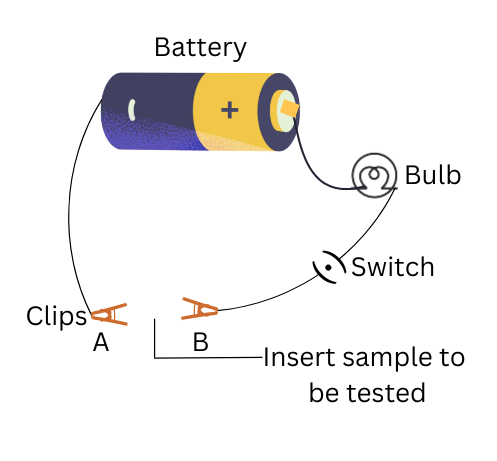
(i) Set up the electric circuit.
(ii) Place the samples of carbon, sulphur and iodine between the terminals of the circuit and note the observations.
Observations:
(i) Coal and sulphur are not shiny while iodine appears shiny.

(ii) Coal cannot be cut with a knife, whereas sulphur and iodine can be cut easily with a knife.

(iii) When beaten with the hammer coal, sulphur and iodine all break into pieces.
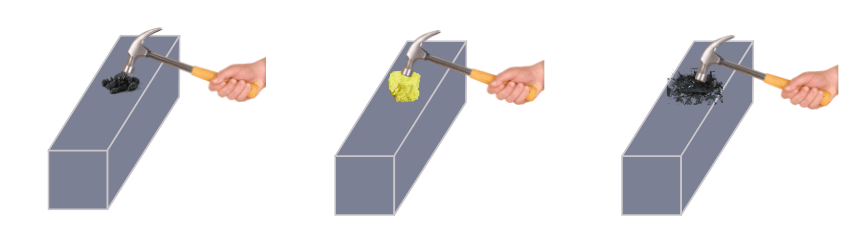
(iv) Coal, sulphur and iodine are not found as wires.
(v) When placed between the terminals of the electric cell, the bulb does not glow in case of all three – coal, sulphur and iodine.
Conclusions:
- In general non-metals are not lustrous, but there are exceptions like iodine.
- In general non-metals are soft, but there are exceptions like coal.
- Non-metals are not malleable.
- Non-metals are not ductile.
- Non-metals do not do not conduct electricity.
Compile your observations regarding metals and non-metals in Table 3.1.
| Element | Symbol | Type of surface | Hardness | Malleability | Ductility | Conducts Electricity | Sonority |
| Iron | Fe | Lustrous | Hard | Yes | Yes | Yes | Yes |
| Copper | Cu | Lustrous | Hard | Yes | Yes | Yes | Yes |
| Aluminium | Al | Lustrous | Hard | Yes | Yes | Yes | Yes |
| Magnesium | Mg | Lustrous | Hard | Yes | Yes | Yes | Yes |
| Sodium | Na | Non-lustrous | Soft | Yes | Yes | Yes | No |
| Zinc | An | Lustrous | Hard | Yes | Yes | Yes | Yes |
| Lead | Pb | Lustrous | Hard | Yes | Yes | Yes | No |
| Coal | C | Non-lustrous | Hard | No | No | No | No |
| Sulphur | S | Non-lustrous | Soft | No | No | No | No |
| Iodine | I | Lustrous | Soft | No | No | No | No |
“7. Complete Activity 3.7 (Page 39).
Collect samples of carbon (coal or graphite), sulphur and iodine.
Carry out the Activities 3.1 to 3.4 and 3.6 with these non-metals and record your observations.” – Solved.
Related Links:
Solution to Activity 3.1
Solution to Activity 3.2
Solution to Activity 3.3
Solution to Activity 3.4
Solution to Activity 3.5
Solution to Activity 3.6
Solution to Activity 3.7
Solution to Activity 3.8
Solution to Activity 3.9
Solution to Activity 3.10
Solution to Activity 3.11
Solution to Activity 3.12
Solution to Activity 3.13
Solution to Activity 3.14


