14. Complete Activity 2.14 (Page 29).
- Collect the following salt samples – sodium chloride, potassium nitrate, aluminium chloride, zinc sulphate, copper sulphate, sodium acetate, sodium carbonate and sodium hydrogencarbonate (some other salts available can also be taken).
- Check their solubility in water (use distilled water only).
- Check the action of these solutions on litmus and find the pH using a pH paper.
- Which of the salts are acidic, basic or neutral?
- Identify the acid or base used to form the salt.
- Report your observations in Table 2.4.
Answer:
Table 2.4:
| Salt | Solubility | Action on litmus | pH | Nature | Acid used | Base used |
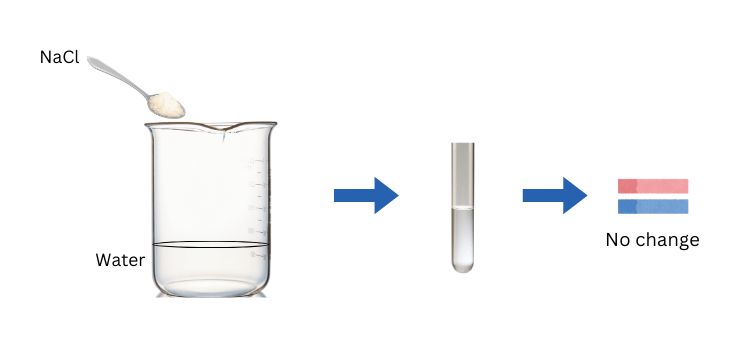
| Sodium chloride | Soluble | No effect | 7 | Neutral | Hydrochloric acid | Sodium hydroxide |
| Potassium nitrate | Soluble | No effect | 7 | Neutral | Nitric acid | Potassium hydroxide |
| Aluminium chloride | Soluble | Blue litmus red | 2.5 | Acidic | Hydrochloric acid | Aluminium hydroxide |
| Zinc sulphate | Soluble | Blue litmus red | 4 | Acidic | Sulphuric acid | Zinc hydroxide |
| Copper sulphate | Soluble | Blue litmus red | 3 | Acidic | Sulphuric acid | Copper hydroxide |
| Sodium acetate | Soluble | Red litmus blue | 8 | Basic | Acetic acid | Sodium hydroxide |
| Sodium carbonate | Soluble | Red litmus blue | 11.5 | Basic | Carbonic acid | Sodium hydroxide |
| Sodium hydrogencarbonate | Soluble | Red litmus blue | 8.5 | Basic | Carbonic acid | Sodium hydroxide |
The procedure is shown with sodium chloride as an example:
“14. Complete Activity 2.14 (Page 29).
Collect the following salt samples – sodium chloride, potassium nitrate, aluminium chloride, zinc sulphate, copper sulphate, sodium acetate, sodium carbonate and sodium hydrogencarbonate (some other salts available can also be taken).
Check their solubility in water (use distilled water only).
Check the action of these solutions on litmus and find the pH using a pH paper.
Which of the salts are acidic, basic or neutral?
Identify the acid or base used to form the salt.
Report your observations in Table 2.4.” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 2.1
Solution to Activity 2.2
Solution to Activity 2.3
Solution to Activity 2.4
Solution to Activity 2.5
Solution to Activity 2.6
Solution to Activity 2.7
Solution to Activity 2.8
Solution to Activity 2.9
Solution to Activity 2.10
Solution to Activity 2.11
Solution to Activity 2.12
Solution to Activity 2.13
Solution to Activity 2.14
Solution to Activity 2.15


