1. Complete Activity 2.1 (Page 18).
- Collect the following solutions from the science laboratory hydrochloric acid (HCl), sulphuric acid (H2 SO4), nitric acid (HNO3), acetic acid (CH3COOH), sodium hydroxide (NaOH), calcium hydroxide [Ca(OH)2 ], potassium hydroxide (KOH), magnesium hydroxide [Mg(OH)2], and ammonium hydroxide (NH4OH).
- Put a drop of each of the above solutions on a watch-glass one by one and test with a drop of the indicators shown in Table 2.1.
- What change in colour did you observe with red litmus, blue litmus, phenolphthalein and methyl orange solutions for each of the solutions taken?
- Tabulate your observations in Table 2.1.
Answer:
Aim: To test the given solutions using the indicators and conclude based on the changes in colour of the indicators.
Materials Required: Hydrochloric acid (HCl), sulphuric acid (H2 SO4), nitric acid (HNO3), acetic acid (CH3COOH), sodium hydroxide (NaOH), calcium hydroxide [Ca(OH)2], potassium hydroxide (KOH), magnesium hydroxide [Mg(OH)2], and ammonium hydroxide (NH4OH, red litmus, blue litmus, phenolphthalein and methyl orange solutions.
Some of the acids are shown below:

Some of the bases are shown below:
Procedure:
(i) One drop of each of the given solutions is put on the watch-glass one by one and tested with a drop of the indicators.
(ii) The change in colour with red litmus, blue litmus, phenolphthalein and methyl orange solutions for each of the solutions taken is tabulated in Table 2.1.
Observations:
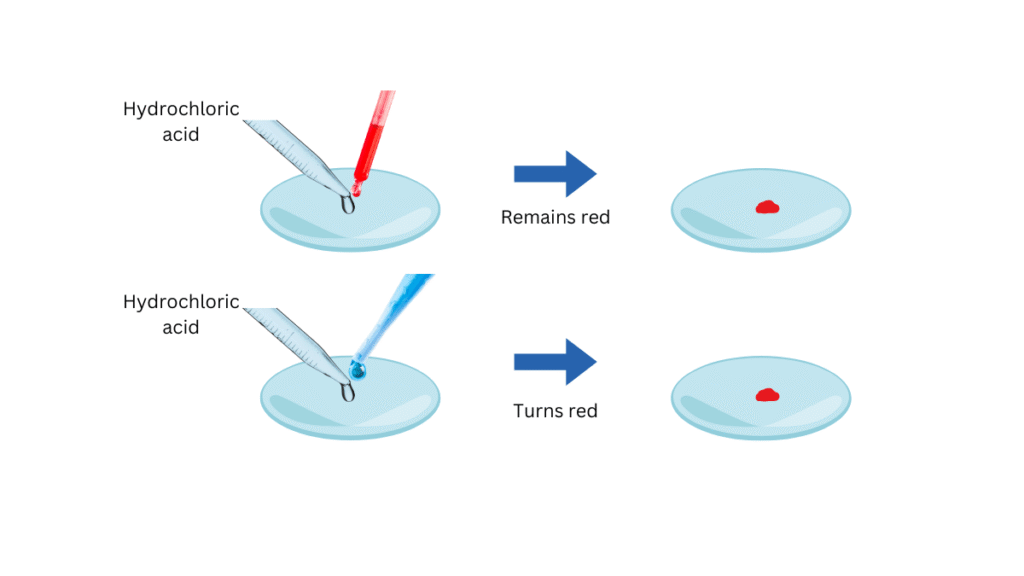
Table 2.1
| Sample solution | Red litmus solution | Blue litmus solution | Phenolphthalein solution | Methyl orange solution |
| Hydrochloric acid (HCl) | No change | Turns red | Colourless | Turns red |
| Sulphuric acid (H2 SO4) | No change | Turns red | Colourless | Turns red |
| Nitric acid (HNO3) | No change | Turns red | Colourless | Turns red |
| Acetic acid (CH3COOH) | No change | Turns red | Colourless | Turns red |
| Sodium hydroxide (NaOH) | Turns blue | No change | Turns pink | Turns yellow |
| Calcium hydroxide [Ca(OH)2] | Turns blue | No change | Turns pink | Turns yellow |
| Potassium hydroxide (KOH) | Turns blue | No change | Turns pink | Turns yellow |
| Magnesium hydroxide [Mg(OH)2] | Turns blue | No change | Turns pink | Turns yellow |
| Ammonium hydroxide (NH4OH) | Turns blue | No change | Turns pink | Turns yellow |
Conclusions:
- Hydrochloric acid (HCl), sulphuric acid (H2 SO4), nitric acid (HNO3), acetic acid (CH3COOH) are acids.
- Sodium hydroxide (NaOH), calcium hydroxide [Ca(OH)2], potassium hydroxide (KOH), magnesium hydroxide [Mg(OH)2], and ammonium hydroxide (NH4OH) are bases.
- Acids do not change the colour of red litmus solution but turn blue litmus solution to red.
- Bases do not change the colour of blue litmus solution but turn red litmus solution to blue.
- Phenolphthalein solution remains colourless in acidic solution and turns pink in contact with a basic solution.
- Methyl orange turns red in contact with an acidic solution and turns yellow in contact with a basic solution.
“1. Complete Activity 2.1 (Page 18).
Tabulate your observations in Table 2.1.
Collect the following solutions from the science laboratory hydrochloric acid (HCl), sulphuric acid (H2 SO4), nitric acid (HNO3), acetic acid (CH3COOH), sodium hydroxide (NaOH), calcium hydroxide [Ca(OH)2 ], potassium hydroxide (KOH), magnesium hydroxide [Mg(OH)2], and ammonium hydroxide (NH4OH).
Put a drop of each of the above solutions on a watch-glass one by one and test with a drop of the indicators shown in Table 2.1.
What change in colour did you observe with red litmus, blue litmus, phenolphthalein and methyl orange solutions for each of the solutions taken?” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 2.1
Solution to Activity 2.2
Solution to Activity 2.3
Solution to Activity 2.4
Solution to Activity 2.5
Solution to Activity 2.6
Solution to Activity 2.7
Solution to Activity 2.8
Solution to Activity 2.9
Solution to Activity 2.10
Solution to Activity 2.11
Solution to Activity 2.12
Solution to Activity 2.13
Solution to Activity 2.14
Solution to Activity 2.15


