CAUTION: This Activity needs the teacher’s assistance.
- Collect the samples of the same metals as in Activity 3.9.
- Put small pieces of the samples separately in beakers half-filled with cold water.
- Which metals reacted with cold water? Arrange them in the increasing order of their reactivity with cold water.
- Did any metal produce fire on water?
- Does any metal start floating after some time?
- Put the metals that did not react with cold water in beakers half-filled with hot water.
- For the metals that did not react with hot water, arrange the apparatus as shown in Fig. 3.3 and observe their reaction with steam.
- Which metals did not react even with steam?
- Arrange the metals in the decreasing order of reactivity with water.
Answer:
Aim:
Materials Required: Sodium, calcium, magnesium, aluminium, zinc, iron, copper, lead, beakers, cold water, hot water, beakers, two laboratory stands, two glass tubes, glass wool soaked in water, cork, delivery tube.
Procedure:
(i) Put a piece of sodium, calcium, magnesium, aluminium, zinc, iron, copper, lead separately in beakers filled with cold water. Note the observations.
(ii) Put the metals that showed no reaction with cold water in beakers half-filled with hot water. Note the observations.
(iii) For the metal samples that showed no reaction with hot water, set up the apparatus shown in the figure and observe which metals react with steam.
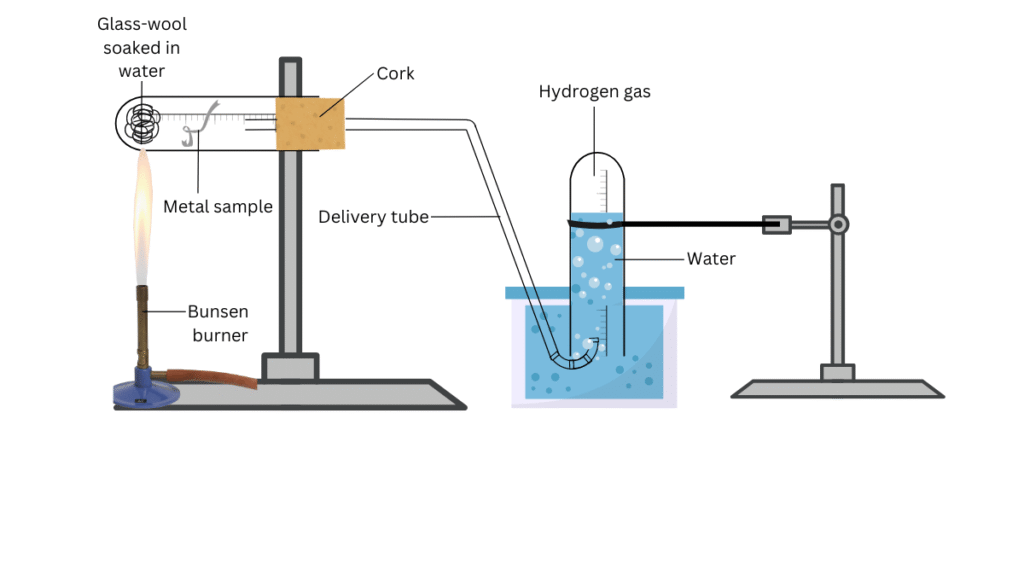
(iv) Finally, arrange the metals in the decreasing order of reactivity with water.
Observations:
Reaction with Cold Water: Sodium reacted violently with cold water and produced a fire. Calcium also reacted with cold water, although the reaction was less violent that sodium.Calcium started floating due to bubbles of gas sticking to its surface, indicating that a reaction had taken place. Magnesium, aluminium, zinc, iron, copper, lead did no react with cold water.
Reaction with Hot Water: Magnesium reacted with hot water and started floating due to bubbles of gas sticking to its surface. Aluminium, zinc, iron, copper, lead did not react with cold water.
Reaction with Steam: Aluminium, zinc, iron reacted with steam to evolve a gas which could be seen displacing water in the inverted glass tube in apparatus.
No reaction: Lead and copper showed no reaction with water at all.
Conclusions: The metals in the decreasing order of reactivity with water are: Sodium > calcium > magnesium > aluminium, zinc, iron > lead, copper. The complete order of reactivity is not possible to determine using this experiment as we do not know the relative rates of the reactions.
“CAUTION: This Activity needs the teacher’s assistance.
- Collect the samples of the same metals as in Activity 3.9.
- Put small pieces of the samples separately in beakers half-filled with cold water.
- Which metals reacted with cold water? Arrange them in the increasing order of their reactivity with cold water.
- Did any metal produce fire on water?
- Does any metal start floating after some time?
- Put the metals that did not react with cold water in beakers half-filled with hot water.
- For the metals that did not react with hot water, arrange the apparatus as shown in Fig. 3.3 and observe their reaction with steam.
- Which metals did not react even with steam?
- Arrange the metals in the decreasing order of reactivity with water.” – Solved.
Related Links:
Solution to Activity 3.1
Solution to Activity 3.2
Solution to Activity 3.3
Solution to Activity 3.4
Solution to Activity 3.5
Solution to Activity 3.6
Solution to Activity 3.7
Solution to Activity 3.8
Solution to Activity 3.9
Solution to Activity 3.10
Solution to Activity 3.11
Solution to Activity 3.12
Solution to Activity 3.13
Solution to Activity 3.14


