4. Complete 2.4 (Page 20).
• Divide the class into two groups. Give 5 g of iron filings and 3 g of sulphur powder in a china dish to both the groups.
Group I
• Mix and crush iron filings and sulphur powder.
Group II
• Mix and crush iron filings and sulphur powder. Heat this mixture strongly till red hot. Remove from flame and let the mixture cool.
Groups I and II
• Check for magnetism in the material obtained. Bring a magnet near the material and check if the material is attracted towards the magnet.
• Compare the texture and colour of the material obtained by the groups.
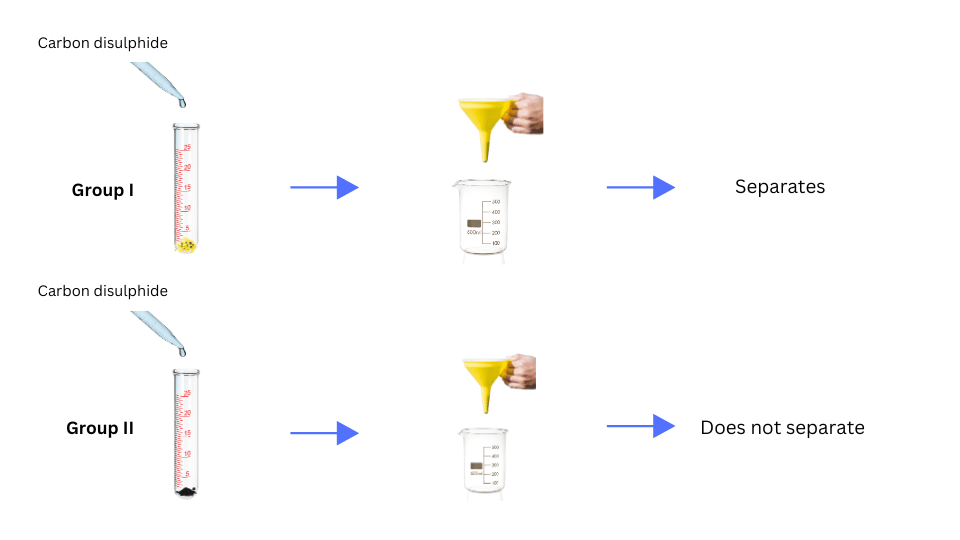
• Add carbon disulphide to one part of the material obtained. Stir well and filter.
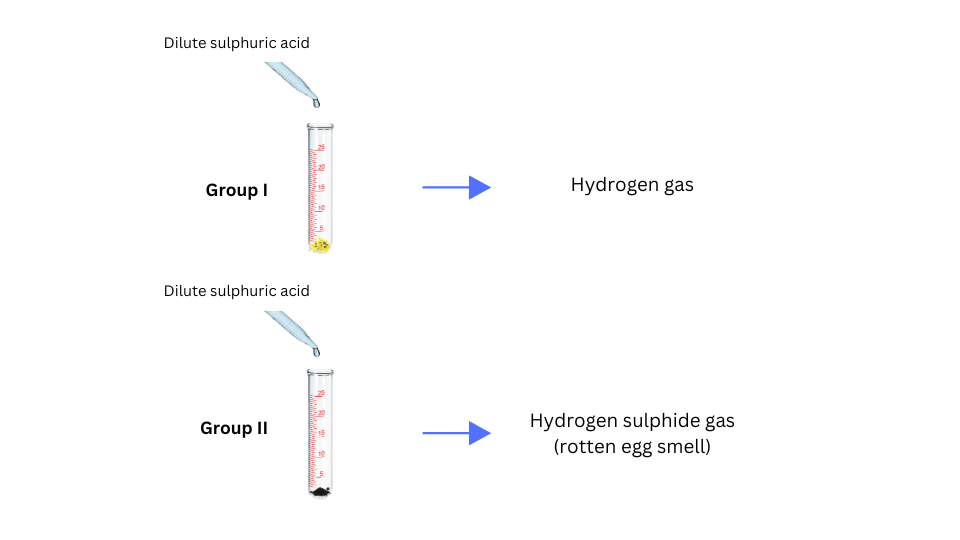
• Add dilute sulphuric acid or dilute hydrochloric acid to the other part of the material obtained. (Note: teacher supervision is necessary for this activity).
• Perform all the above steps with both the elements (iron and sulphur) separately.
Answer:
Aim: To explore the properties of the products obtained by Group I and Group II and conclude based on that.
Materials Required: Iron filings, sulphur powder, china dish, burner, magnet, dilute sulphuric acid.
Procedure:
(i) The class was divided into two groups.
(ii) 5 g of iron filings and 3 g of sulphur powder were provided in a china dishto both Group I and Group II.
(iii) Students in Group I crushed and mixed the iron filings and sulphur powder.
(iv) Students in Group II crushed and mixed the iron filings and sulphur powder. Then the mixture was heated by them till red hot, removed from the flame and cooled.
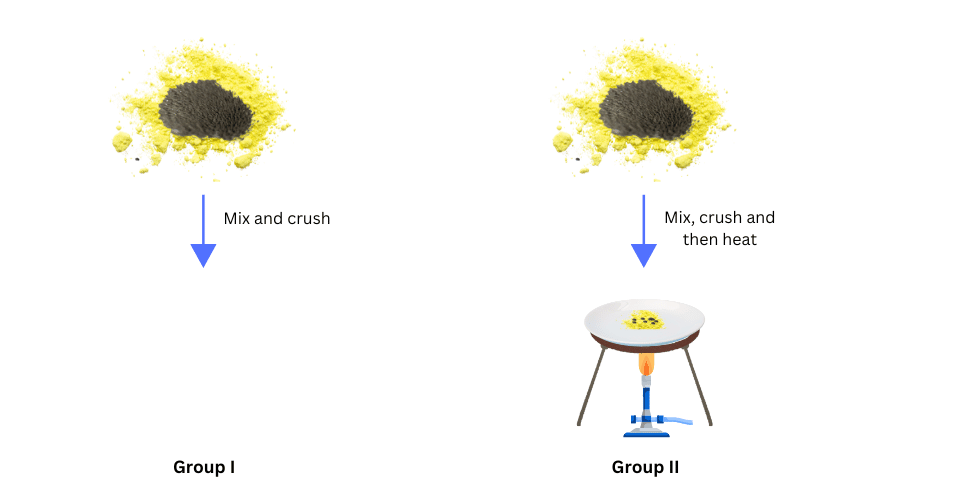
(v) Both Group I and Group II brought a magnet near their sample and noted the observations.
(vi) Both Group I and Group IIcompared the texture and colour of their samples.
(vii) Both Group I and Group II added carbon disulphide to one part of their sample. The solutions were stirred well and filtered using the filtration apparatus. The observations were noted.
(viii) Both Group I and Group II added dilute sulphuric acid to the other part of the sample. The observations were noted.
(ix) The groups performed all the above steps with both the elements (iron and sulphur) separately.
Observations:
- The texture of the material obtained by Group I was non-uniform and the colour was yellow and black. The texture of the material obtained by Group II was uniform and the colour was black.
- The material obtained by Group I was magnetic. The material obtained by group II was non-magnetic.
- When carbon disulphide was added to the material obtained by Group I and the mixture was filtered, iron filings residue was left on the filter paper and got separated from the sulphur dissolved in carbon disulphide, which passed through. When carbon disulphide was added to the material obtained by Group II, the components could not be separated.
- When dilute sulphuric acid was added, Group I obtained a colourless and odourless gas and Group II obtained a colourless gas with the smell of rotten eggs.
- When the groups performed all the steps with iron and sulphur separately, iron showed magnetic behaviour, sulphur was dissolved in carbon disulphide and when dilute sulphuric acid was added to iron, a colourless and odourless gas was obtained.
Conclusions:
- The material obtained by Group I is a mixture of the two substances, iron and sulphur. We conclude that the texture of mixtures is non-uniform and the properties were the same as the constituent substances, which was confirmed when iron and sulphur were tested separately.
- The material obtained by Group II is a compound. We conclude that the composition and colour of compounds is uniform and properties are different than the constituent substances.
- In case of mixtures the constituents can be separated easily using physical methods. In case of compounds the constituents cannot be separated easily using physical methods.
- The gases obtained on addition of sulphuric acid were different, further proving that the chemical properties of mixtures and compounds are different, even though they are formed from the same substances.
Solutions to Group Activity
Solution to Activity 2.1
Solution to Activity 2.2
Solution to Activity 2.3
Solutions to Chapter 2 Is Matter Around Us Pure?


