Answer:
Aim: To observe what happens to the potassium permanganate crystal in the hot water and cold water and conclude.
Materials Required: Potassium permanganate crystal, hot water, cold water, two glasses.
Procedure:
(i) Fill the first glass with cold water and the second glass with hot water.
(ii) Drop a crystal of potassium permanganate into the glass of cold water and another into the glass of hot water. Do this without stirring and allow the crystals to settle at the bottom. Note the observations.
Observations:
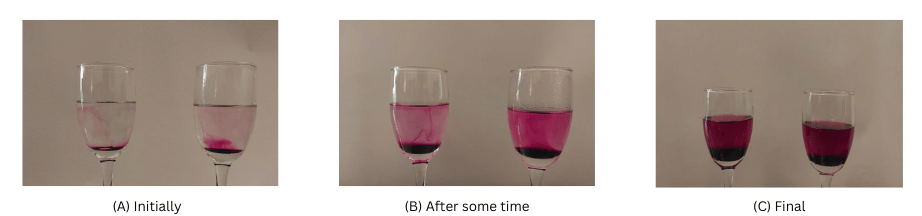
- In case of the glass with cold water, there is initially nothing just above the solid crystal in the glass. In case of the glass of hot water, there is a layer of purple just above the solid crystal.
- As time passes the blue colour spreads through the water. This is slow for cold water and fast for the hot water.
In the images below, the glass with cold water is to the left and the glass with hot water is to the right.
Conclusions:
- This suggests that the particles of solid and liquid possess kinetic energy and hence, are in constant motion.
- The particles of solid diffuse into the spaces between the particles of liquid and this intermixing happens on its own.
- The particles of permanganate diffused faster in hot water due to high kinetic energy due to high temperature.

Related Links:
Solution to Group Activity
Solution to Activity 1.1
Solution to Activity 1.2
Solution to Activity 1.3
Solution to Activity 1.4
Solution to Activity 1.6
Solution to Activity 1.7
Solution to Activity 1.8
Solution to Activity 1.9
Solution to Activity 1.10
Solution to Activity 1.11
Solution to Activity 1.12
Solution to Activity 1.13
Solution to Activity 1.14
Solutions to Chapter 1 Matter in Our Surroundings


