11. Complete Activity 3.11 (Page No: 33). Fill the two cans used in Activity 3.10 with the same amount of hot water at the same temperature (say, at 600 C). Leave the cans in a room or in a shade. Note the temperature of water after 10-15 minutes. Does the temperature of water in both the cans fall by the same amount?
Answer: Find the details of the experiment below:
Aim: To find in which container – black or white – has a faster rate of cooling, and based on your observation provide an explanation.
Materials Required: Two containers – the outer surface of one is painted black and the outer surface of another is painted white, hot water at 600 C, thermometer, shaded room.
Procedure:
(i) The container with the outer surface painted black and the container with the outer surface painted white are filled with equal amounts of water at 600 C.
(ii) Leave the container in a shaded room.
(iii) Note the temperature of the water after 10-15 minutes.
Observation:
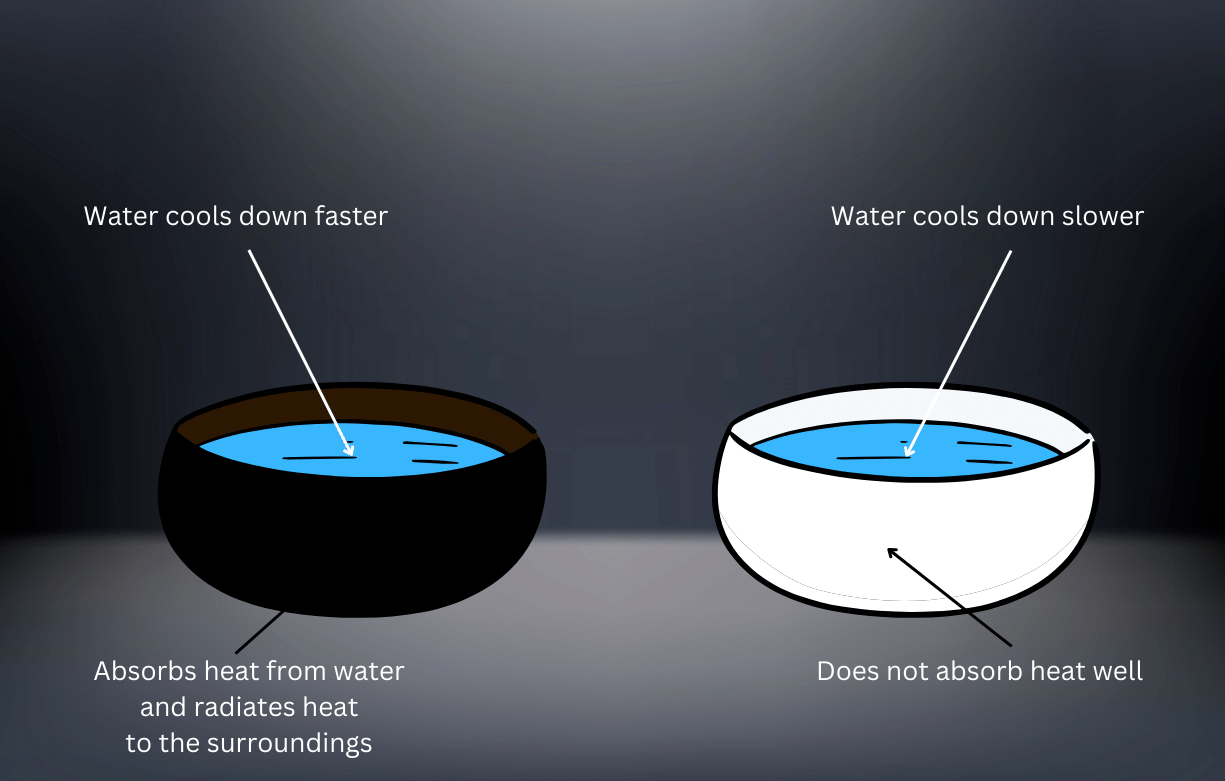
You will notice that the temperature of the water in the black container will fall by a greater amount than the temperature of the water in the white container, that is the water in the black container will be cooler.
Conclusion:
The hot water in both containers will radiate heat into the surroundings. However, the colour of outer surface of the two containers makes the difference:
The black surface of the first container is much better at absorbing heat than the white surface of the second container. Therefore, the black surface absorbs heat from the hot water by conduction and sends it into the surroundings by radiation, which brings the water temperature down quickly. However, the white surface will not be able to absorb the heat and will reflect most of the heat, due to which the water temperature inside does not fall that much. Therefore, we conclude that black surfaces absorb and radiate heat well. White surfaces reflect most of the heat and are not good absorbers of heat.
Related Links:
Solution to Extended Learning Problem 1
Solution to Extended Learning Problem 2
Solution to Extended Learning Problem 3
Solution to Extended Learning Problem 4
Solution to Extended Learning Problem 5
Solution to Activity 3.1
Solution to Activity 3.2
Solution to Activity 3.3
Solution to Activity 3.4
Solution to Activity 3.5
Solution to Activity 3.6
Solution to Activity 3.7
Solution to Activity 3.8
Solution to Activity 3.9
Solution to Activity 3.10
Solutions to Chapter 3 Heat


