6. Complete Activity 5.6 (Page 49). Get a small piece of a thin strip or ribbon of magnesium. Clean its tip with sandpaper. Bring the tip near a candle flame. It burns with a brilliant white light (Fig. 5.3). When it is completely burnt it leaves behind a powdery ash. Does the ash look like the magnesium ribbon? Collect the ash and mix it with a small amount of water. Stir the mixture (aqueous solution) well. Test the mixture with blue and red litmus papers. Does the mixture turn red litmus blue? Does the mixture turn blue litmus red? On the basis of this test, how do you classify the aqueous solution — acidic or basic?
Answer:
Answer: The above activity can be carried out as follows:
Aim: To observe the changes when you burn the magnesium ribbon and mix the ash with water and based on that make the necessary conclusions.
Materials Required: Magnesium ribbon, sandpaper, candle, water, blue and red litmus paper.
Procedure:
(i) Clean the tip of the magnesium ribbon with sandpaper.
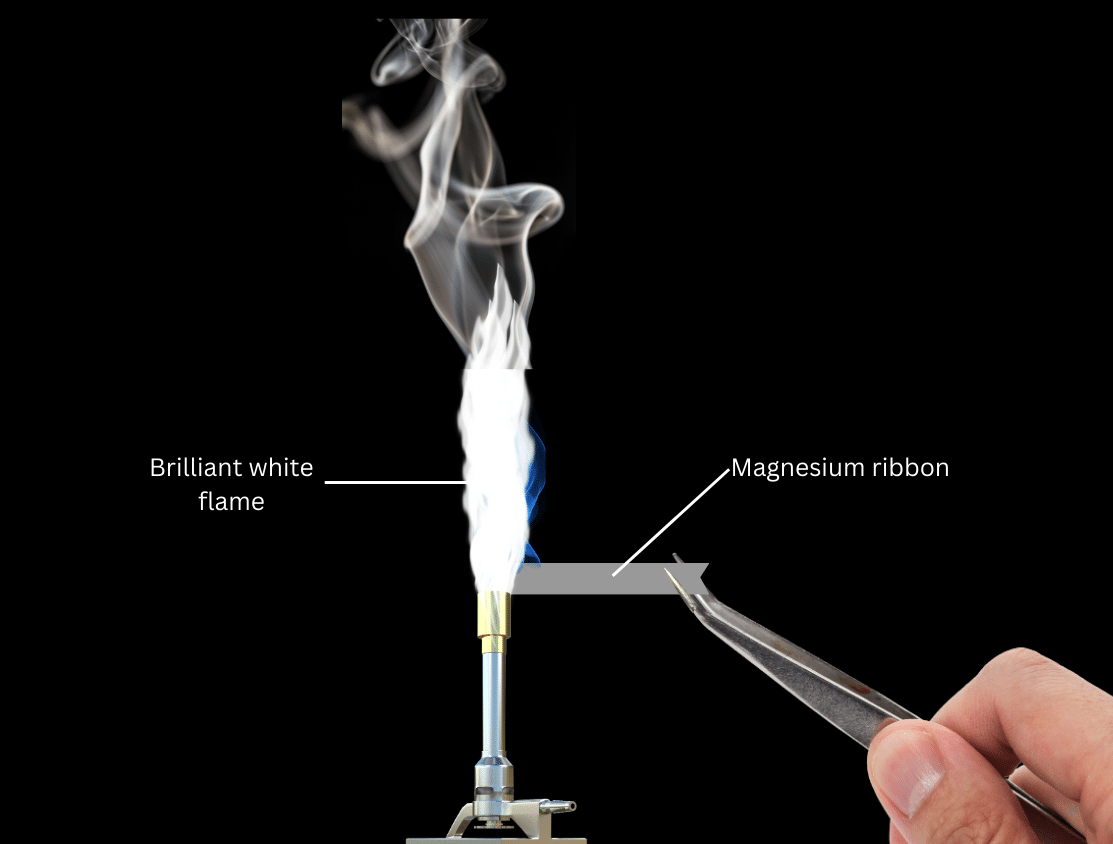
(ii) Light a candle or Bunsen burner and hold the magnesium ribbon directly on the flame with a pair of tweezers. Observe what happens. (Caution: do not stare too long directly at the flame, it can harm your eyes)
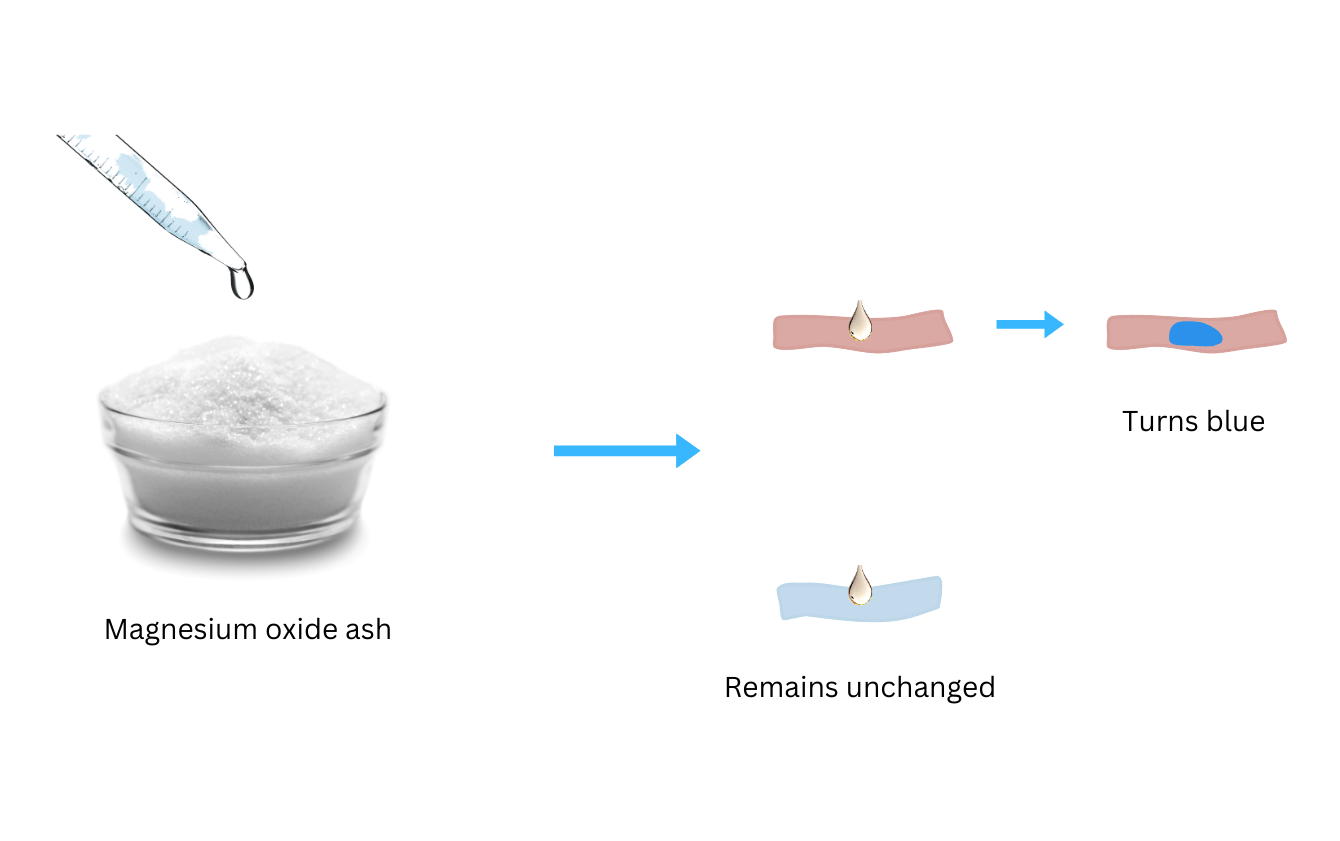
(iii) Mix the ash thus obtained with water and stir the mixture well.
(iv) Test the mixture with blue and red litmus paper and observe what happens.
Observations:
The magnesium ribbon burns with a brilliant white flame and a white, powdery ash forms, which is quite different from shiny, metallic magnesium ribbon.
- When you test the ash-water mixture with red and blue litmus paper, the solution only changes red litmus to blue.
Conclusions:
We conclude that:
- When magnesium ribbon burns in air it forms a white, powdery ash called magnesium oxide. This is a new substance with different properties, hence it is a chemical change. The equation is shown below:
Magnesium (Mg) + Oxygen (O2) → Magnesium oxide (MgO)
- When the aqueous solution of ash and water is tested with blue and red litmus, it turns only red litmus blue. This is because the base magnesium hydroxide is formed. Once again, a new substance with different properties is formed. Hence, this is a chemical change. The equation is shown below:
Magnesium oxide (MgO) + Water (H2O) → Magnesium hydroxide [Mg(OH)2]
“Complete Activity 5.6 (Page 49). Get a small piece of a thin strip or ribbon of magnesium. Clean its tip with sandpaper. Bring the tip near a candle flame. It burns with a brilliant white light (Fig. 5.3). When it is completely burnt it leaves behind a powdery ash. Does the ash look like the magnesium ribbon? Collect the ash and mix it with a small amount of water. Stir the mixture (aqueous solution) well. Test the mixture with blue and red litmus papers. Does the mixture turn red litmus blue? Does the mixture turn blue litmus red? On the basis of this test, how do you classify the aqueous solution — acidic or basic?” – Solved.
Related Links:
Solution to Extended Learning – Activities and Projects Question 1
Solution to Extended Learning – Activities and Projects Question 2
Solution to Extended Learning – Activities and Projects Question 3
Solution to Extended Learning – Activities and Projects Question 4
Solution to Activity 5.1
Solution to Activity 5.2
Solution to Activity 5.3
Solution to Activity 5.4
Solution to Activity 5.5
Solution to Activity 5.7
Solution to Activity 5.8
Solutions to Chapter 5 Physical and Chemical Changes


