12. Complete Activity 1.11 (Page 12).
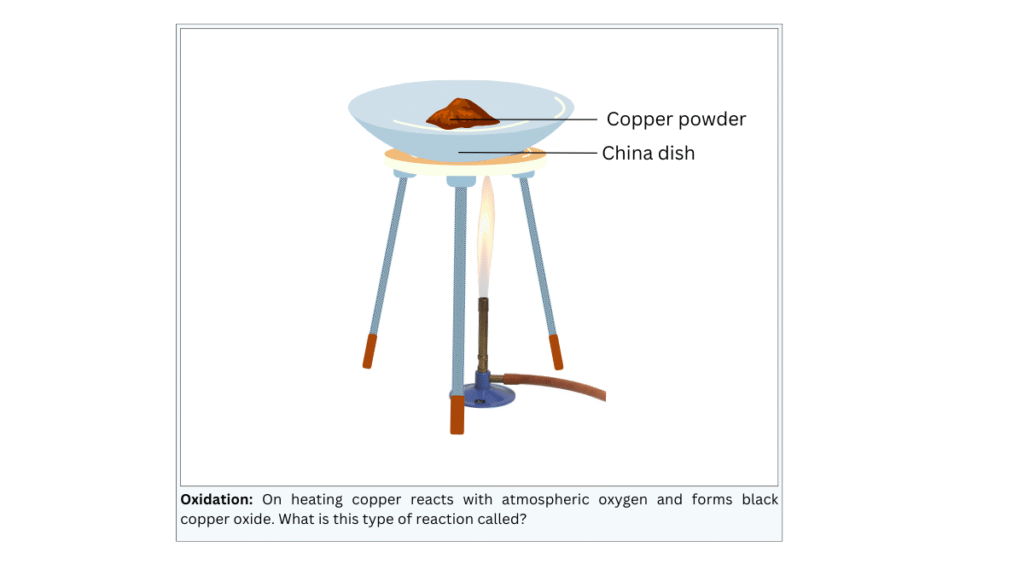
- Heat a china dish containing about 1 g copper powder (Fig. 1.10).
- What do you observe?
Answer:
Aim: To heat 1 g copper powder in the china dish and observe what happens and conclude.
Materials Required: China dish, 1 g copper powder.
Procedure:
(i) Put 1 g copper powder in the china dish. (ii) Heat the china dish using the burner. Note the observations.
Observations:
The surface of the copper powder becomes black.
Conclusions:
- Black copper(II) oxide has formed on the surface of the copper powder due to addition of oxygen to copper.
- This phenomenon is called oxidation and the reaction, called oxidation reaction, is shown below:
2Cu + O2 + Heat —> 2CuO
“12. Complete Activity 1.11 (Page 12).
What do you observe?
Heat a china dish containing about 1 g copper powder (Fig. 1.10).” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 1.1
Solution to Activity 1.2
Solution to Activity 1.3
Solution to Activity 1.4
Solution to Activity 1.5
Solution to Activity 1.6
Solution to Activity 1.7
Solution to Activity 1.8
Solution to Activity
Solution to Activity 1.9
Solution to Activity 1.10
Solution to Activity 1.11


