2. Complete Activity 2.2 (Page 15).
• Let us again divide the class into four groups— A, B, C and D.
• Distribute the following samples to each group:
− Few crystals of copper sulphate to group A.
− One spatula full of copper sulphate to group B.
− Chalk powder or wheat flour to group C.
− Few drops of milk or ink to group D.
• Each group should add the given sample in water and stir properly using a glass rod. Are the particles in the mixture visible?
• Direct a beam of light from a torch through the beaker containing the mixture and observe from the front. Was the path of the beam of light visible?
• Leave the mixtures undisturbed for a few minutes (and set up the filtration apparatus in the meantime). Is the mixture stable or do the particles begin to settle after some time?
• Filter the mixture. Is there any residue on the filter paper?
• Discuss the results and form an opinion.
Answer:
Aim: For each mixture we need to determine if the particles are visible, if the path of light is visible when a torch is shone on it, if it is stable and if there is any residue on the filter paper during filtration and make conclusions based on that.
Materials Required: Few crystals of copper sulphate, copper sulphate powder, chalk powder, ink, four beakers, water.
Procedure:
(i) Divide the class into four groups: A, B, C and D.
(ii) Group A should add the few crystals of copper sulphate in water and stir properly using a glass rod.
(iii) Group B should add the spatula full of copper sulphate in water and stir properly using a glass rod.
(iv) Group C should add chalk powder to water and stir properly. (v) Group D should add ink to water and stir properly.
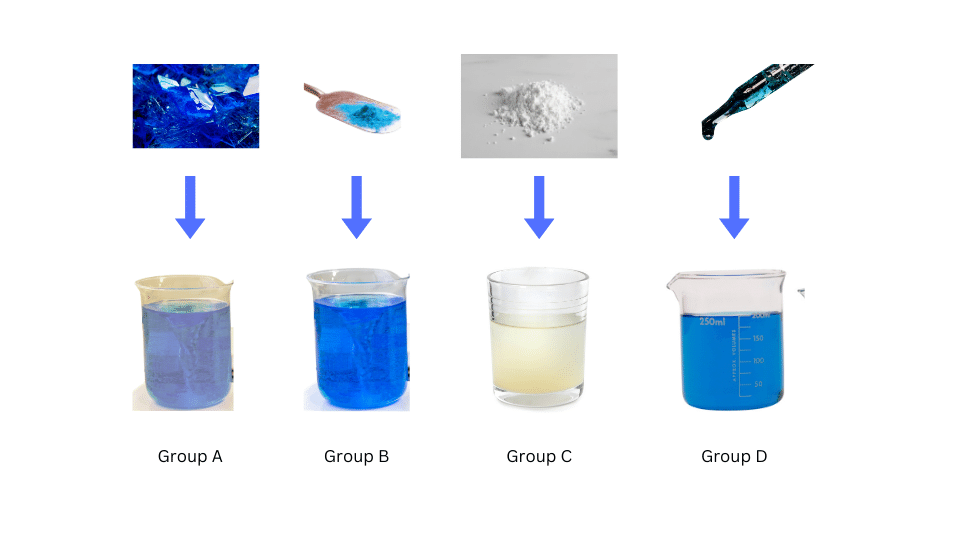
(vi) The sizes of the particles in each mixture are noted.
(vii) For each mixture shine a beam of light using a torch through the beaker and observe from the front if the path of the beam of light is visible.

(viii)Leave the mixtures undisturbed and observe if the particles begin to settle down after sometime.
(ix) Prepare a filtration apparatus, filter each mixture and see if there is any residue left on the filter paper.

(x) Discuss the results and form an opinion.
Observations:
Group A:
- Particles in the copper sulphate solution are not visible.
- When a torch is shone on the contents of the beaker, the path of light is not visible.
- When left undisturbed for a few minutes, the particles do not settle.
- When filtered there is no residue left on the filter paper.
Group B:
- Particles in the copper sulphate solution are not visible.
- When a torch is shone on the contents of the beaker, the path of light is not visible.
- When left undisturbed for a few minutes, the particles do not settle.
- When filtered there is no residue left on the filter paper.
Group C:
- Particles of chalk powder are visible.
- When a torch is shone on the contents of the beaker, the path of light is visible.
- When left undisturbed for a few minutes, the particles settle.
- When filtered, there is chalk powder left on the filter paper as residue.
Group D:
- Particles of ink cannot be seen with naked eyes.
- When a torch is shone on the contents of the beaker, the path of light is visible.
- When left undisturbed for a few minutes, the particles do not settle.
- When filtered there is no residue left on the filter paper.
Conclusions:
- Group A and Group B have a solution. A solution is homogeneous and particles cannot be seen with the naked eye. Solutions do not exhibit Tyndall effect (do not scatter light) and are stable. The solute particles of a solution are too small to be separated by filtration.
- Group C has a suspension. A suspension is heterogeneous and particles can be seen with the naked eye. It exhibits Tyndall effect (scatters light) and is not stable. The solute particles can be separated by filtration.
- Group D has a colloidal solution. A colloidal solution is heterogeneous and cannot be seen with the naked eye. It exhibits Tyndall effect (scatters light) and is stable. The solute particles are too small to be separated by filtration.
“Activity 2.2 (Page 15).
• Let us again divide the class into four groups— A, B, C and D.
• Distribute the following samples to each group:
− Few crystals of copper sulphate to group A.
− One spatula full of copper sulphate to group B.
− Chalk powder or wheat flour to group C.
− Few drops of milk or ink to group D.
• Each group should add the given sample in water and stir properly using a glass rod. Are the particles in the mixture visible?
• Direct a beam of light from a torch through the beaker containing the mixture and observe from the front. Was the path of the beam of light visible?
• Leave the mixtures undisturbed for a few minutes (and set up the filtration apparatus in the meantime). Is the mixture stable or do the particles begin to settle after some time?
• Filter the mixture. Is there any residue on the filter paper?
• Discuss the results and form an opinion.” – Solved.
Solutions to Group Activity
Solution to Activity 2.1
Solution to Activity 2.3
Solution to Activity 2.4
Solutions to Chapter 2 Is Matter Around Us Pure?


