1. Complete Activity 2.1 (Page 14).
• Let us divide the class into groups A, B, C and D.
• Group A takes a beaker containing 50 mL of water and one spatula full of copper sulphate powder. Group B takes 50 mL of water and two spatula full of copper sulphate powder in a beaker.
• Groups C and D can take different amounts of copper sulphate and potassium permanganate or common salt (sodium chloride) and mix the given components to form a mixture.
• Report the observations on the uniformity in colour and texture.
Answer:
Aim: To observe the uniformity in colour and textures for the mixtures and conclude based on that.
Materials Required: Two beakers, water, spatula, copper sulphate powder, potassium permanganate.
Procedure:
(i) Divide the class into four groups: A, B, C and D.
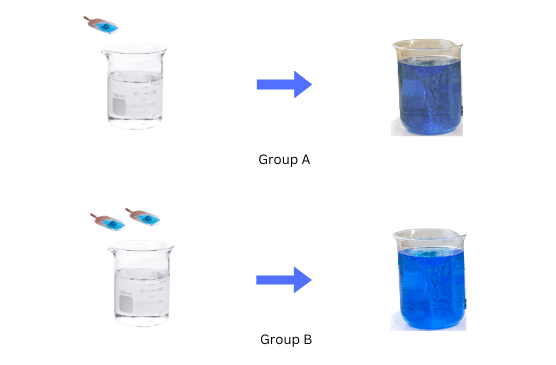
(ii)Group A dissolves one spatula full of copper sulphate powder in 50 mL of water in the first beaker.
(iii) Group B dissolves two spatulas full of copper sulphate powder in 50 mL of water in the second beaker.
(iv) Group C takes a certain amount (3g) of copper sulphate and a certain amount (5g) of potassium permanganate and mixes them together.
(v) Group D takes a certain amount (5g) of copper sulphate and a certain amount (3g) of potassium permanganate and mixes them together.
(vi) Report the observations on the uniformity in colour and texture in each case.
Observations:
For Group A and Group B the mixtures have uniform composition throughout. However, the colour of the mixture for Group B is more intense than the colour of the mixture for Group A.
The mixtures for Group A and Group D have physically distinct parts and are non-uniform.

Conclusions:
- The mixtures for Groups A and B have uniform composition throughout and are called homogeneous mixtures.
- There is more copper sulphate powder in the mixture for Group B, which the makes the colour more intense. This shows that homogeneous mixtures can have variable composition.
- The mixtures for Groups C and D have physically distinct parts and have non-uniform compositions. Hence, they are called heterogeneous mixtures.
“Activity 2.1 (Page 14).
• Let us divide the class into groups A, B, C and D.
• Group A takes a beaker containing 50 mL of water and one spatula full of copper sulphate powder. Group B takes 50 mL of water and two spatula full of copper sulphate powder in a beaker.
• Groups C and D can take different amounts of copper sulphate and potassium permanganate or common salt (sodium chloride) and mix the given components to form a mixture.
• Report the observations on the uniformity in colour and texture. ” – Solved.
Solutions to Group Activity
Solution to Activity 2.2
Solution to Activity 2.3
Solution to Activity 2.4
Solutions to Chapter 2 Is Matter Around Us Pure?


