Hello and welcome students to NCERT Solutions to Class 7 Science Curiosity Chapter 2! Our solutions are written in a logical and easy-to-understand manner to help you grasp the concepts easily. Moreover, our Class 7 Science Curiosity Chapter 2 Exploring Substances: Acidic, Basic, and Neutral solutions will be regularly updated with new content and extra materials, so make sure you keep visiting!
Happy studying!
Let Us Enhance Our Learning (Class 7 Science Curiosity Chapter 2):
1. A solution turns the red litmus paper to blue. Excess addition of which of the following solution would reverse the change?
(i) Lime water
(ii) Baking soda
(iii) Vinegar
(iv) Common salt solution
Answer: (iii) Vinegar
The initial solution is a basic solution which turns red litmus paper to blue. Excess addition of acidic vinegar solution will reverse the change (turn blue litmus to red) as there will be excess acid left over after neutralising the effect of the base.
2. You are provided with three unknown solutions labelled A, B, and C, but you do not know which of these are acidic, basic, or neutral. Upon adding a few drops of red litmus solution to solution A, it turns blue. When a few drops of turmeric solution are added to solution B, it turns red. Finally, after adding a few drops of red rose extract to solution C, it turns green.
Based on the observations, which of the following is the correct sequence for the nature of solutions A, B, and C?
(i) Acidic, acidic, and acidic
(ii) Neutral, basic, and basic
(iii) Basic, basic, and acidic
(iv) Basic, basic, and basic
Answer: (iv) Basic, basic, and basic
Solution A is a basic solution because it turns red litmus to blue.
Solution B is a basic solution because it turns turmeric solution to red.
Solution C is a basic solution because it turns china rose indicator to green.
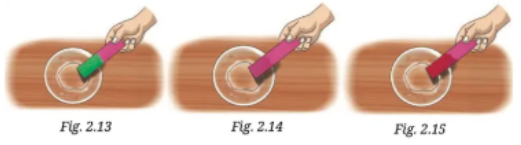
3. Observe and analyse Figs. 2.13, 2.14, and 2.15, in which red rose extract paper strips are used. Label the nature of solutions present in each of the containers.
Answer:
Fig. 2.13 has a basic solution as it turns china rose indicator green.
Fig. 2.14 has a neutral solution as it does not change the colour of china rose indicator.
Fig. 2.15 has an acidic solution as it turns china rose indicator reddish.
4. A liquid sample from the laboratory was tested using various indicators:

Based on the tests, identify the acidic or basic nature of the liquid and justify your answer.
Answer:
Based on the tests,the liquid sample is acidic in nature because it turned blue litmus red and showed no colour change with turmeric indicator.
5. Manya is blindfolded. She is given two unknown solutions to test and determine whether they are acidic or basic. Which indicator should Manya use to test the solutions and why?
Answer:
Manya should use olfactory indicators such as onion strips to test the unknown solutions to determine whether they are acidic or basic.
6. Could you suggest various materials which can be used for writing the message on the white sheet of paper (given at the beginning of the chapter) and what could be in the spray bottle? Make a table of various possible combinations and the colour of the writing obtained.
Answer:
| Material used to write message | Material in Spray Bottle | Colour of writing |
| Soap solution (Basic) | Turmeric solution | Red |
| Lime water (Basic) | Turmeric solution | Red |
| Orange juice (Acidic) | Red rose extract | Red |
| Baking soda (Basic) | Red rose extract | Green |
| Vinegar (Acidic) | Blue litmus solution | Red |
| Amla juice (Acidic) | Blue litmus solution | Red |
7. Grape juice was mixed with red rose extract; the mixture got a tint of red colour. What will happen if baking soda is added to this mixture? Justify your answer.
Answer:
When baking soda is added to the mixture of grape juice with red rose extract, the red colour of the solution will gradually disappear. This is because baking soda is basic in nature which will neutralise the acidic grape juice. If baking soda is added in excess, the solution will become basic and will become green in colour.
8. Keerthi wrote a secret message to her grandmother on her birthday using orange juice. Can you assist her grandmother in revealing the message? Which indicator would you use to make it visible?
Answer:
Orange juice is acidic in nature. We know that red rose extract turns red in acidic solutions. Hence, I will tell her grandmother to use red rose extract to reveal the secret message, which will be red in colour.
9. How can natural indicators be prepared? Explain by giving an example.
Answer:
We can prepare turmeric indicator which is a natural indicator by the following process:
(i) A spoonful of turmeric (haldi) is taken in a container and a little water is added to make a paste.
(ii) A piece of filter paper is dipped in the turmeric paste until it gets yellow colour.
(iii) It is taken out and allowed to dry.
(iv) This yellow paper is cut into thin strips, which can be used as ‘turmeric paper’
10. Three liquids are given to you. One is vinegar, another is a baking soda solution, and the third is a sugar solution. Can you identify them only using turmeric paper? Explain.
Answer:
Baking soda will turn turmeric paper red because baking soda is a basic substance. The other two liquids, vinegar and sugar, do not change the colour of turmeric paper and hence cannot be distinguished.
11. The extract of red rose turns the liquid X to green. What will the nature of liquid X be? What will happen when excess of amla juice is added to liquid X?
Answer:
Liquid X is basic in nature because it turns red rose extract green. When excess of acidic amla juice is added to liquid X, the green colour of the solution will become red. This is because the excess acid will neutralise the effect of the base and make the solution acidic. Hence, the colour of the solution changes to red.
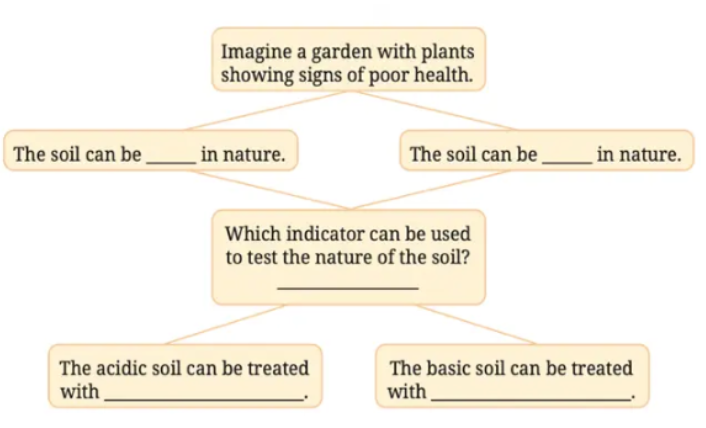
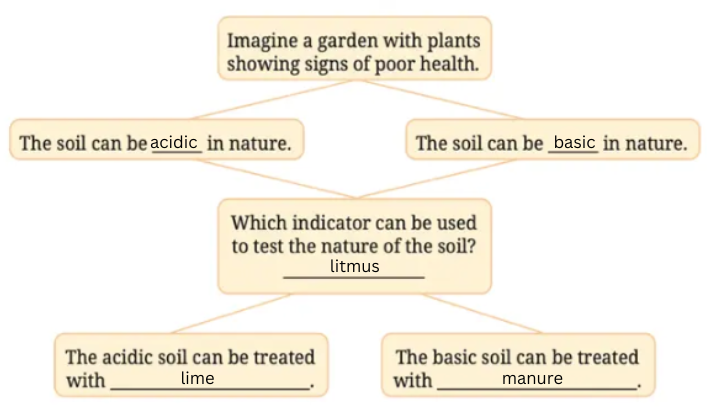
12. Observe and analyse the information given in the following flowchart. Complete the missing information.
Answer:
Hope you enjoyed our solutions material on Class 7 Science Curiosity Chapter 2! Click on the links below to access the solutions to the remaining chapters!
Related Links:
Chapter 1 The Ever-Evolving World of Science
Chapter 2 Exploring Substances: Acidic, Basic, and Neutral
Chapter 3 Electricity: Circuits and their Components
Chapter 4 The World of Metals and Non-metals
Chapter 5 Changes Around Us: Physical and Chemical
Chapter 6 Adolescence: A Stage of Growth and Change
Chapter 7 Heat Transfer in Nature
Chapter 8 Measurement of Time and Motion
Chapter 9 Life Processes in Animals
Chapter 10 Life Processes in Plants
Chapter 11 Light: Shadows and Reflections
Chapter 12 Earth, Moon, and the Sun


