Hello students! Here we provide you with NCERT Solutions to Class 7 Science Curiosity Chapter 4 Metals and Non-Metals! This is an easy chapter to score from if you understand and study it well! So, take the opportunity and plunge in! Our NCERT Solutions to Class 7 Science Curiosity Chapter 4 Metals and Non-Metals will definitely help you with your classwork and prepare you well for your exams!
Take care and good luck!
Let Us Enhance Our Learning (Class 7 Science Curiosity Chapter 4):
1. Which metal is commonly used to make food packaging materials as it is cheaper, and its tin sheets can be folded easily into any shape?
(i) Aluminium
(ii) Copper
(iii) Iron
(iv) Gold
Answer: (i) Aluminium
Explanation: Aluminium metal foil is commonly used to make food packaging materials as it is cheaper and can be folded easily into any shape due to its malleability.
2. Which of the following metal catches fire when it comes in contact with water?
(i) Copper
(ii) Aluminium
(iii) Zinc
(iv) Sodium
Answer: (iv) Sodium
Explanation: Sodium is a metal which is stored in kerosene because it reacts vigorously with oxygen and water, thereby catching fire.
3. State with reason(s) whether the following statements are True [T] or False [F].
(i) Aluminium and copper are examples of non-metals used for making utensils and statues. [ ]
(ii) Metals form oxides when combined with oxygen, the solution of which turns blue litmus paper to red. [ ]
(iii) Oxygen is a non-metal essential for respiration. [ ]
(iv) Copper vessels are used for boiling water because they are good conductors of electricity. [ ]
Answer:
(i) [F] False. Aluminium and copper are examples of metals.
(ii) [F] False. When combined with oxygen, metals form basic oxides, the solution of which turns red litmus paper blue.
(iii) [T] True. Oxygen is a non-metal essential for respiration.
(iv) [F] False. Copper vessels are used for boiling water because they are good conductors of heat and hence the heat gets transferred to the water inside the vessels.
4. Why are only a few metals suitable for making jewellery?
Answer:
Metals used in jewellery must have certain essential properties, including lustre, malleability, ductility, and resistance to corrosion and. Gold and silver are metals that possess all these characteristics, which is why they are suitable for making jewellery.
5. Match the uses of metals and non-metals given in Column I with the jumbled names of metals and non-metals given in Column II.
| Column I | Column II |
| (i) Used in electrical wiring | (a) E N X Y G O |
| (ii) Most malleable and ductile | (b) N E C O H I R L |
| (iii) Living organisms cannot survive without it. | (c) P E P O R C |
| (iv) Plants grow healthy when fertilisers containing it are added to the soil. | (d) T E N G O I N R |
| (v) Used in water purification. | (e) O G D L |
Answer:
| Column I | Column II |
| (i) Used in electrical wiring | (a) COPPER |
| (ii) Most malleable and ductile | (e) GOLD |
| (iii) Living organisms cannot survive without it. | (a) OXYGEN |
| (iv) Plants grow healthy when fertilisers containing it are added to the soil. | (d) NITROGEN |
| (v) Used in water purification. | (e) CHLORINE |
6. What happens when oxygen reacts with magnesium and sulfur. What are the main differences in the nature of products formed?
Answer:
Magnesium metal reacts with oxygen to form magnesium oxide. Sulfur (non-metal) reacts with oxygen to form sulfur dioxide.
When magnesium oxide is dissolved in water, it forms a basic solution which turns red litmus paper blue. However, when sulfur dioxide is dissolved in water, it forms an acidic solution called sulfurous acid which turns blue litmus paper red.
7. Complete the following flow chart:
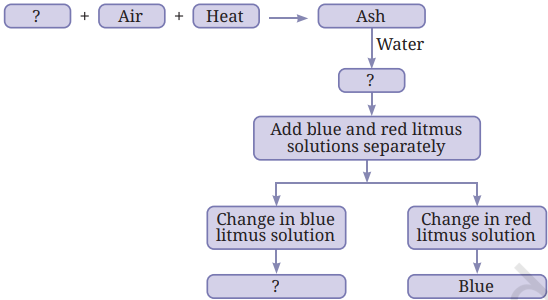
Answer:
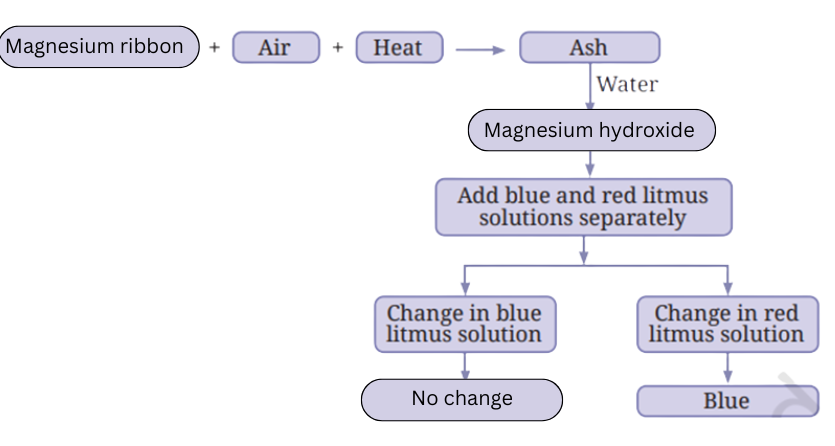
8. You are provided with the following materials. Discuss which material would be your choice to make a pan that is most suitable for boiling water and why?
Iron, copper, sulfur, coal, plastic, wood, cardboard
Answer:
Iron, copper will be the most suitable. This is because iron and copper are good conductors of heat and will be most suitable for boiling water by allowing heat to pass through the pan into the water inside it.
Sulfur, coal, plastic, wood, cardboard are bad conductors of heat and hence, are not suitable for boiling water.
9. You are provided with three iron nails, each dipped in oil, water and vinegar. Which iron nail will not rust, and why?
Answer:
The iron nail dipped in oil will not rust. This is because the layer of oil prevents oxygen and moisture from reaching the surface of the iron nail. Since both oxygen and moisture are needed for rusting to take place, the iron nail will not rust.
10. How do the different properties of metals and non-metals determine their uses in everyday life?
Answer:
Metals:
- Heat conducting ability of metals enable them to be used as heating pans, cooking vessels.
- Electricity conducting ability of metals enable them to be used as electrical wires.
- Metals are malleable and hence they are used to produce jewellery by beating them into different shapes.
- Metals are ductile and hence can be drawn into wires such as in suspension bridges.
- Metals are hard and hence used to build tools and structures.
- Metals are sonorous and hence used to make calling bells etc.
- Metals are lustrous and hence used in decoration and jewellery.
Non-metals:
- The non-metal oxygen is essential for breathing.
- Carbon is essential in everyday life because it is the building block of all life forms. It is a key component of proteins, fats, and carbohydrates, which are necessary for growth and energy.
- Nitrogen is used in the manufacturing of fertilisers and other chemicals. It is an essential nutrient for the growth of plants.
- Chlorine is a non-metal commonly used in water purification.
- Solution of iodine, a non-metal, is applied on wounds as an antiseptic.
11. One of the methods of protecting iron from getting rusted is to put a thin coating of zinc metal over it. Since sulfur does not react with water, can it be used for this purpose? Justify your answer.
Answer:
No, sulfur cannot be used for this purpose. This is because it is brittle and breaks into pieces and hence cannot form a durable and continuous coating like zinc metal.
12. An ironsmith heats iron before making tools. Why is heating necessary in this process?
Answer:
An ironsmith heats iron before making tools to make it soft and hence more malleable. The hot and soft iron can easily be beaten into various shapes for making tools.
Hope you enjoyed our solutions material on Class 7 Science Curiosity Chapter 4! Click on the links below to access the solutions to the remaining chapters!
Related Links:
Chapter 1 The Ever-Evolving World of Science
Chapter 2 Exploring Substances: Acidic, Basic, and Neutral
Chapter 3 Electricity: Circuits and their Components
Chapter 4 The World of Metals and Non-metals
Chapter 5 Changes Around Us: Physical and Chemical
Chapter 6 Adolescence: A Stage of Growth and Change
Chapter 7 Heat Transfer in Nature
Chapter 8 Measurement of Time and Motion
Chapter 9 Life Processes in Animals
Chapter 10 Life Processes in Plants
Chapter 11 Light: Shadows and Reflections
Chapter 12 Earth, Moon, and the Sun


