Play a game for writing formulae.
Example 1 : Make placards with symbols and valencies of the elements separately. Each student should hold two placards, one with the symbol in the right hand and the other with the valency in the left hand. Keeping the symbols in place, students should criss-cross their valencies to form the formula of a compound.
Example 2 : A low cost model for writing formulae: Take empty blister packs of medicines. Cut them in groups, according to the valency of the element, as shown in the figure. Now, you can make formulae by fixing one type of ion into other.
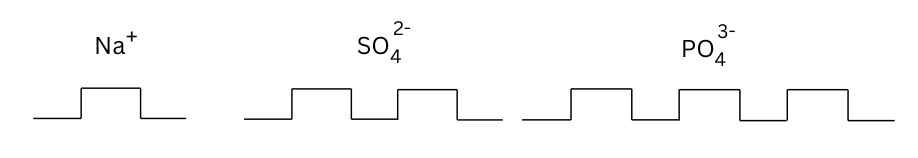
Answer:
Example 1:
Aim: To form formulae of compounds using the placards.
Materials Required: Placards, pen.
Procedure:
(i) With the pen write the symbols of several elements and their respective valencies separately on the placards.
(ii) Each student should hold the placard with the symbol of the element on the right hand and the placard with the valency of the element on the left hand.
(iii) Keeping the symbols in place, students should criss-cross their valencies to form the formula of a compound.
Observations:
The exercise for the compound CaCl2 is shown below:
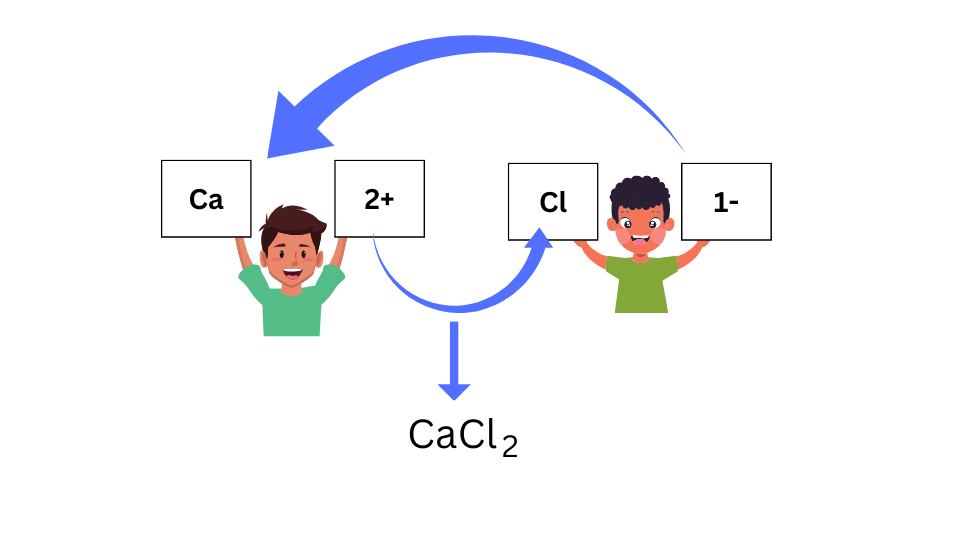
Conclusions:
The valency is the combining power of the element. In the above example, the valency of Ca is 2. Hence, Ca has the capacity of combining with 2 Cl atoms.
Example 2:
Aim: To make formulae using empty blister packs of medicines.
Materials Required: Empty medicine blister packs, scissors.
Procedure:
(i) Cut the empty blister packs of medicines in groups, according to the valency of the element.
(ii) Make formulae by fixing one type of ion onto another.
(iii) Do this activity for sodium sulphate and sodium phosphate.
Observations:
The formulae of sodium sulphate can be found as follows:
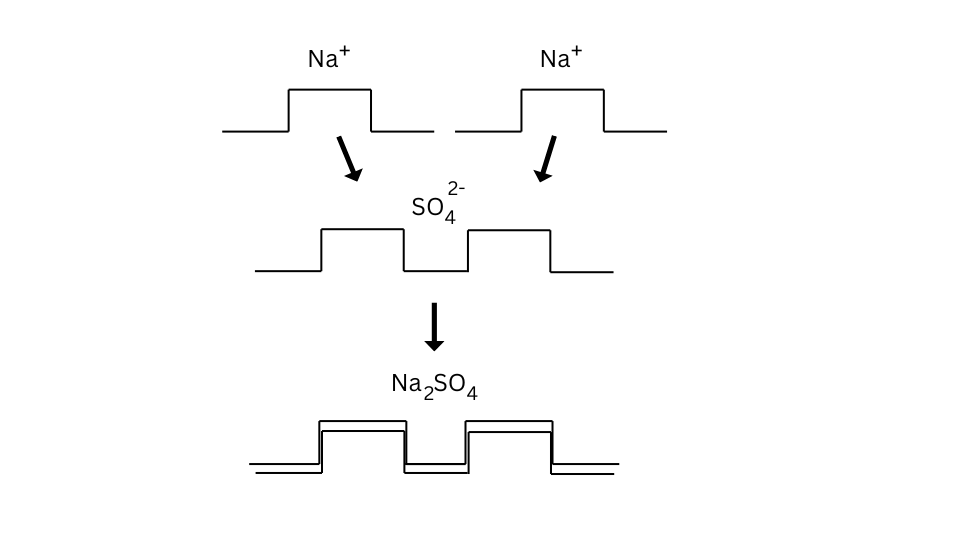
The formulae of sodium phosphate can be found as follows:
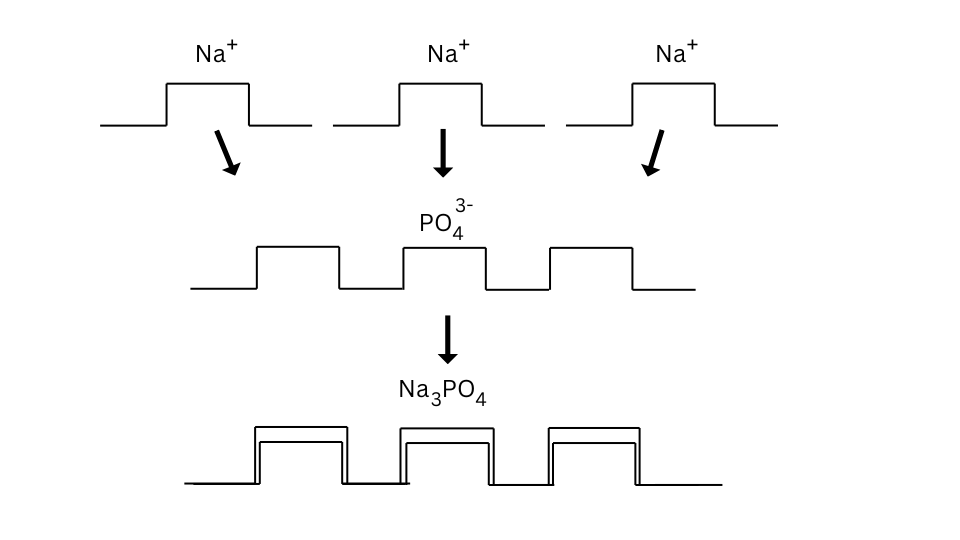
Conclusions:
- The valency is the combining power of the element.
- In case of sodium sulphate the valency of sulphate ion was 2, hence it combined with two sodium atoms.
- In case of sodium phosphate the valency of phosphate ion was 3, hence it combined with three sodium atoms.
“Play a game for writing formulae.
Example 1 : Make placards with symbols and valencies of the elements separately. Each student should hold two placards, one with the symbol in the right hand and the other with the valency in the left hand. Keeping the symbols in place, students should criss-cross their valencies to form the formula of a compound.
Example 2 : A low cost model for writing formulae: Take empty blister packs of medicines. Cut them in groups, according to the valency of the element, as shown in the figure. Now, you can make formulae by fixing one type of ion into other.” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 3.1
Solution to Activity 3.2
Solutions to Chapter 3 Atoms and Molecules


