(I) Prepare your own indicator
- Crush beetroot in a mortar.
- Add sufficient water to obtain the extract.
- Filter the extract by the procedure learnt by you in earlier classes.
- Collect the filtrate to test the substances you may have tasted earlier.
- Arrange four test tubes in a test tube stand and label them as A,B,C and D. Pour 2 mL each of lemon juice solution, soda-water, vinegar and baking soda solution in them respectively.
- Put 2-3 drops of the beetroot extract in each test tube and note the colour change if any. Write your observation in a Table.
- You can prepare indicators by using other natural materials like extracts of red cabbage leaves, coloured petals of some flowers such as Petunia, Hydrangea and Geranium.
Answer:
Aim: To observe the colour change of beetroot extract in lemon juice solution, soda-water, vinegar and baking soda solutionand make the necessary conclusions.
Materials Required: Mortar and pestle, beetroot, water, filtration funnel, filter paper, laboratory stand, beakers, 4 test tubes, 2 mL each of lemon juice solution, soda-water, vinegar and baking soda solution, dropper.
Procedure:
(i) Crush the beetroot using mortar and pestle.
(ii) Pour the solution in a beaker and added sufficient water to obtain the extract.
(iii) Prepare the filtration apparatus by attaching the filtration funnel (along with the filter paper) to the laboratory stand.
(iv) Then filter the extract and collect it in another beaker.
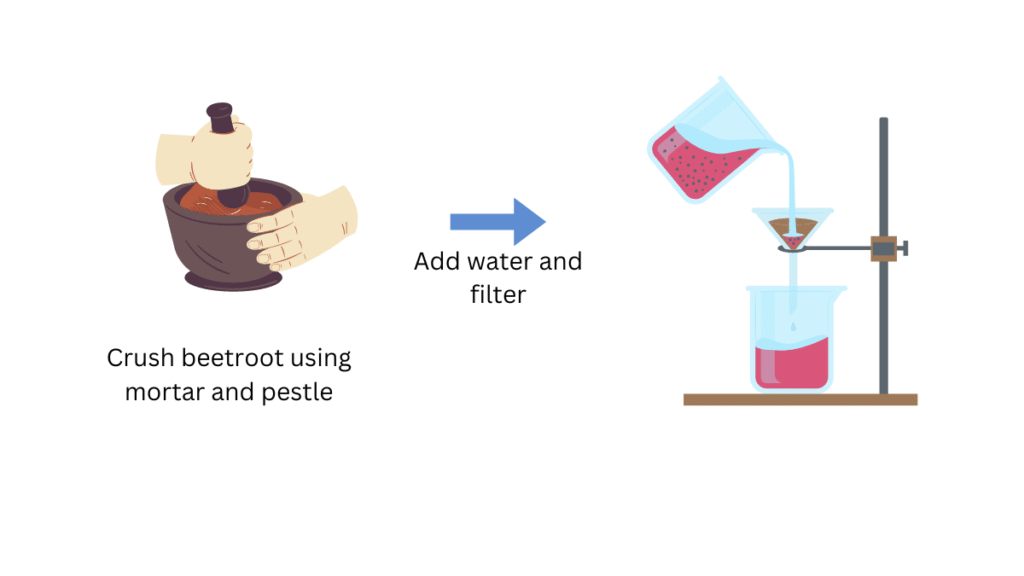
(v) Label the four test tubes as A, B, C and D.
(vi) Pour 2 mL each of lemon juice solution, soda-water, vinegar and baking soda solution in them respectively.
(vii) Put 2-3 drops of the beetroot extract in each test tube and note the observations in a Table.
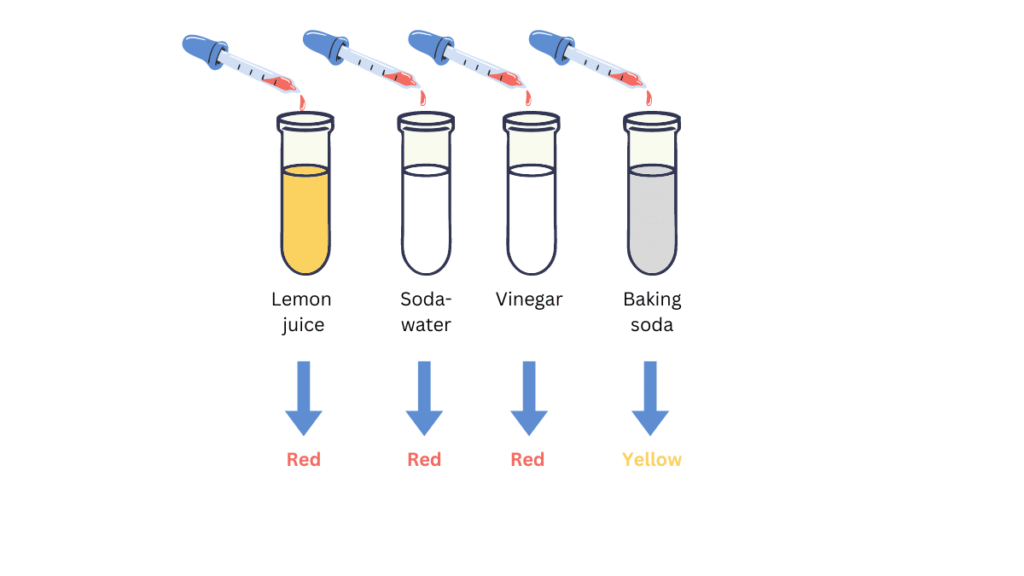
Observations:
| Test tube | Solution | Colour change of beetroot extract |
| A | Lemon juice | Light red |
| B | Soda-water | Dark red |
| C | Vinegar | Dark red |
| D | Baking soda | Yellow |
Conclusions:
Beetroot extract can be used as an indicator because it turns red in acidic solution and yellow in basic solution.
(II) Preparing a soda-acid fire extinguisher
The reaction of acids with metal hydrogencarbonates is used in the fire extinguishers which produce carbon dioxide.
- Take 20 mL of sodium hydrogencarbonate (NaHCO3) solution in a wash-bottle.
- Suspend an ignition tube containing dilute sulphuric acid in the wash-bottle (Fig. 2.10).
- Close the mouth of the wash-bottle.
- Tilt the wash-bottle so that the acid from the ignition tube mixes with the sodium hydrogencarbonate solution below.
- You will notice discharge coming out of the nozzle.
- Direct this discharge on a burning candle. What happens?
Answer:
Aim: To mix the acid with the sodium hydrogencarbonate solution, observe what happens and make the necessary conclusions.
Materials Required: Sodium hydrogencarbonate, dilute sulphuric acid, wash-bottle, ignition tube, thread, candle.
Procedure:
(i) Carefully pour 20 mL of sodium hydrogencarbonate (NaHCO3) solution in the wash-bottle.
(ii) Fill half of the ignition tube with dilute sulphuric acid.
(iii) Now suspend the ignition tube with the thread in the wash-bottle.
(iv) Close the mouth of the wash bottle such that some portion of the thread remains outside.
(v) Now tilt the wash-bottle to mix the dilute sulphuric acid in the ignition tubewith the sodium hydrogencarbonate solution below. Observe what happens.
(vi) Direct the nozzle at the burning candle and observe what happens.
Observations:
- When dilute sulphuric acid in the ignition tubemixeswith the sodium hydrogencarbonate solution, discharge comes out of the nozzle.
- When the discharge is directed on a burning candle, the candle gets extinguished.
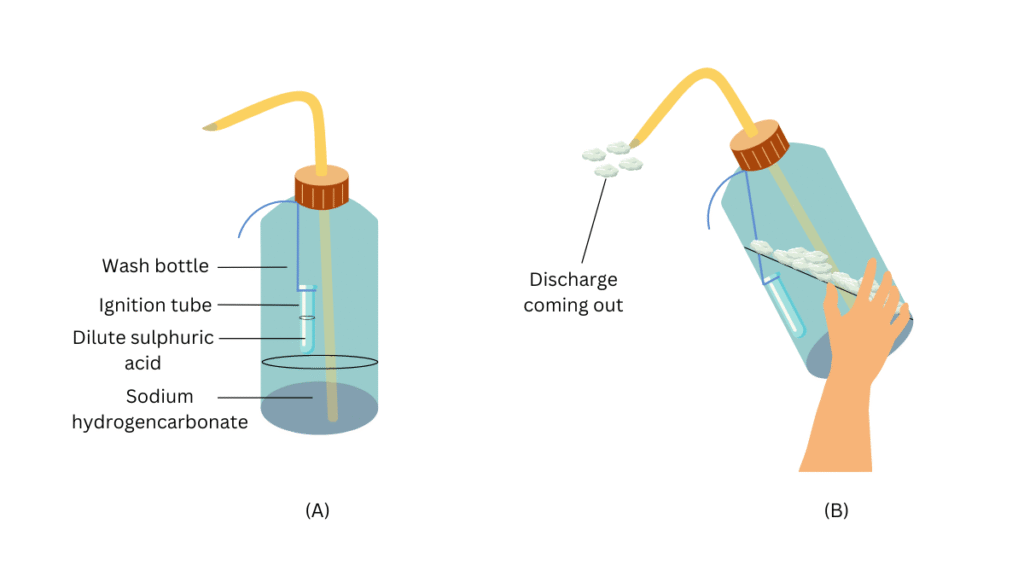
Conclusions:
- The reaction between dilute sulphuric acid and sodium hydrogencarbonate produces sodium sulphate, water and carbon dioxide. The equation is shown below:
H2SO4 + 2NaHCO3 —> Na2SO4 + 2H2O + 2CO2 - The carbon dioxide forms a blanket around the burning flame of the candle and cuts of the oxygen supply, thereby extinguishing the candle.
- Thus, this contraption can be used as a soda-acid fire extinguisher.
Related Links:
Solution to Group Activity
Solution to Activity 2.1
Solution to Activity 2.2
Solution to Activity 2.3
Solution to Activity 2.4
Solution to Activity 2.5
Solution to Activity 2.6
Solution to Activity 2.7
Solution to Activity 2.8
Solution to Activity 2.9
Solution to Activity 2.10
Solution to Activity 2.11
Solution to Activity 2.12
Solution to Activity 2.13
Solution to Activity 2.14
Solution to Activity 2.15


