3. Complete Activity 2.3 (Page 19).
CAUTION: This activity needs the teacher’s assistance.
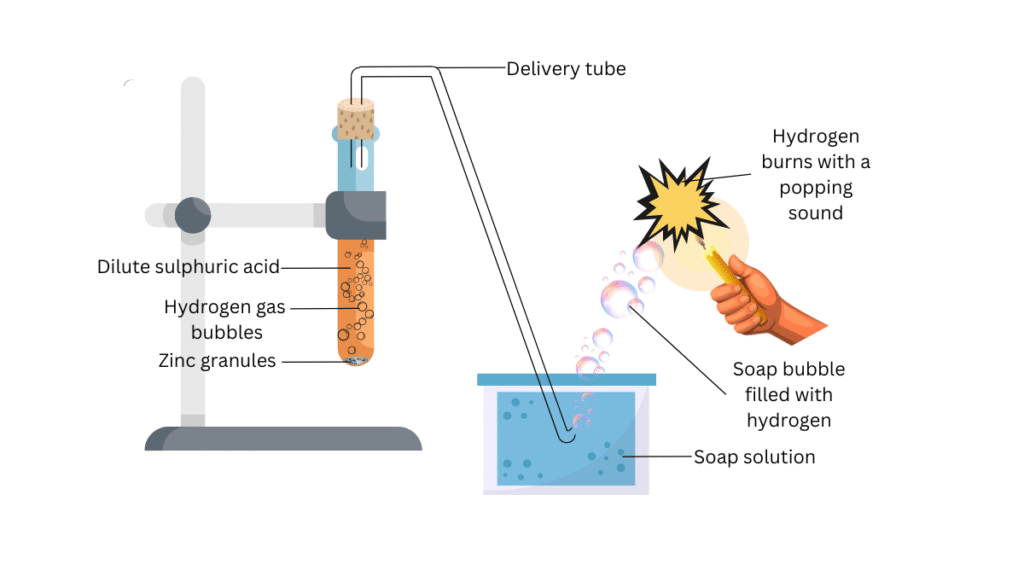
- Set the apparatus as shown in Fig. 2.1.
- Take about 5 mL of dilute sulphuric acid in a test tube and add a few pieces of zinc granules to it.
- What do you observe on the surface of zinc granules?
- Pass the gas being evolved through the soap solution.
- Why are bubbles formed in the soap solution?
- Take a burning candle near a gas filled bubble.
- What do you observe?
- Repeat this Activity with some more acids like HCl, HNO3 and CH3COOH.
- Are the observations in all the cases the same or different?
Answer:
Aim: To hold a burning candle near gas bubbles formed in the reaction between dilute sulphuric acidand zinc and conclude based on the observations. To repeat the same activity with the acids HCl, HNO3 and CH3COOH and conclude based on the observations.
Materials Required: 5 mL of dilute sulphuric acid, zinc granules, laboratory stand, test tube, delivery tube, soap solution, candle.
Procedure:
(i) Set up the apparatus as shown in the figure. Attached the test tube to the laboratory stand. Put the cork on the mouth of the test tube and place the delivery tube through the cork as shown. Now submerge the other end of the delivery tube in the soap solution as shown.
(ii)Open the cork andadd about 5 mL of dilute sulphuric acid in the test tube and add a few pieces of zinc granules to it. Replace the cork and note what happens on the surface of zinc granules.
(iii) Observe what happens in the soap solution.
(iv)Hold the burning candle near a gas filled bubble above the soap solution as shown in the figure and note the observations.
(v) Now repeat this Activity with some the acids HCl, HNO3 and CH3COOH. Note the observations in each case.
Observations:
- Gas bubbles are observed on the surface of zinc granules.
- Bubbles form in the soap solution and rise up into the air.
- The bubbles burn with a popping sound when the candle is brought next to them.
- The observations are the same when the activity is repeated with HCl, HNO3 and CH3COOH.
Conclusions:
- Since the gas bubbles burn with a popping sound, the bubbles are filled with hydrogen gas.
- Acids react with certain metals to form salt and hydrogen gas. The reactions for this activity are shown below:
Zn + H2SO4 —> ZnSO4 + H2
Zn + 2HCl —> ZnCl2 + H2
Zn + 2HNO3 —> Zn(NO3)2 + H2
Zn + CH3COOH → Zn(CH3COO)2 + H2
“3. Complete Activity 2.3 (Page 19).
CAUTION: This activity needs the teacher’s assistance.
Are the observations in all the cases the same or different?
Set the apparatus as shown in Fig. 2.1.
Take about 5 mL of dilute sulphuric acid in a test tube and add a few pieces of zinc granules to it.
What do you observe on the surface of zinc granules?
Pass the gas being evolved through the soap solution.
Why are bubbles formed in the soap solution?
Take a burning candle near a gas filled bubble.
What do you observe?
Repeat this Activity with some more acids like HCl, HNO3 and CH3COOH.” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 2.1
Solution to Activity 2.2
Solution to Activity 2.3
Solution to Activity 2.4
Solution to Activity 2.5
Solution to Activity 2.6
Solution to Activity 2.7
Solution to Activity 2.8
Solution to Activity 2.9
Solution to Activity 2.10
Solution to Activity 2.11
Solution to Activity 2.12
Solution to Activity 2.13
Solution to Activity 2.14
Solution to Activity 2.15


