Welcome students! We are confident that these top-quality solutions to NCERT Class 7 Science Chapter 3 Heat combined with a plethora of additional questions of various formats will definitely answer all your questions and clear your concepts. By answering all textbook exercise questions, extended learning – activities and projects, in-text questions and in-text activities, we have provided you with a complete solutions package. We have approached the topic from all angles to help you grasp the concepts and to show you how to solve unknown questions. These questions will be similar to your exam questions, so make sure you understand them well.
Solutions to Exercise (Page No 24) of NCERT Class 7 Science Chapter 3 Heat:
1. State similarities and differences between the laboratory thermometer and the clinical thermometer.
Answer:
Some of the similarities between the laboratory thermometer and the clinical thermometer are as follows:
- Both measure temperature.
- Both consist of a long, narrow, uniform glass tube with a bulb at one end.
- Both consist of mercury.
- Both consist of bigger marks with a number of smaller marks between the bigger marks which are known as divisions.
- Both use the Celsius scale.
- Both contain a maximum and a minimum temperature.
- Mercury is a toxic substance, so both have to be handled with care to prevent breakage.
Some of the differences between the laboratory thermometer and the clinical thermometer are as follows:
| Clinical thermometer | Laboratory thermometer |
| The range of the Celsius scale is 350C – 420C. | The range of the Celsius scale is -100C – 1100C. |
| Used to measure body temperature only. | Not suitable for measuring body temperature. |
| Cannot measure high temperatures. | Can measure high temperatures. |
| It has a kink which prevents the mercury level from falling back down. | It does not have a kink. |
2. Give two examples each of conductors and insulators of heat.
Answer:
Two examples of conductors of heat are: aluminium, iron. Two examples of insulators of heat are: air, wood.
3. Fill in the blanks:
(a) The hotness of an object is determined by its __________.
(b) Temperature of boiling water cannot be measured by a ___________ thermometer.
(c) Temperature is measured in degree ____________.
(d) No medium is required for transfer of heat by the process of __________.
(e) A cold steel spoon is dipped in a cup of hot milk. Heat is transferred to its other end by the process of ____________.
(f) Clothes of ____________ colours absorb heat better than clothes of light colours.
Answers:
(a) The hotness of an object is determined by its temperature.
(b) Temperature of boiling water cannot be measured by a clinical thermometer.
(c) Temperature is measured in degree Celsius.
(d) No medium is required for transfer of heat by the process of radiation.
(e) A cold steel spoon is dipped in a cup of hot milk. Heat is transferred to its other end by the process of conduction.
(f) Clothes of dark colours absorb heat better than clothes of light colours.
4. Match the following:
| Column-I | Column-II |
| (i) Land breeze blows during | (a) Summer |
| (ii) Sea breeze blows during | (b) Winter |
| (iii) Dark coloured clothes are preferred during | (c) Day |
| (iv) Light coloured clothes are preferred during | (d) Night |
Answer: The correct table is shown below:
| Column-I | Column-II |
| (i) Land breeze blows during | (d) Night |
| (ii) Sea breeze blows during | (c) Day |
| (iii) Dark coloured clothes are preferred during | (b) Winter |
| (iv) Light coloured clothes are preferred during | (a) Summer |
5. Discuss why wearing more layers of clothing during winter keeps us warmer than wearing just one thick piece of clothing.
Answer:
Wearing more layers of clothing during winter keeps us warmer than wearing just one thick piece of clothing because multiple layers of clothing allow air to be trapped inside the layers. Air is an insulator and is a bad conductor of heat. Therefore, it prevents heat from flowing from the body to the surroundings.
6. Look at Fig. 4.13. Mark where the heat is being transferred by conduction, by convection and by radiation.
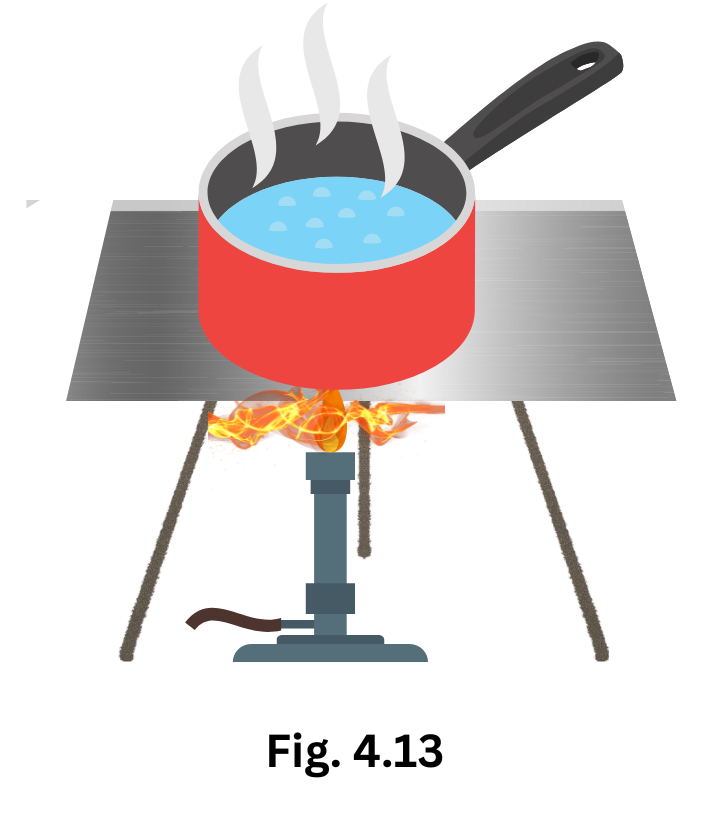
Answer:
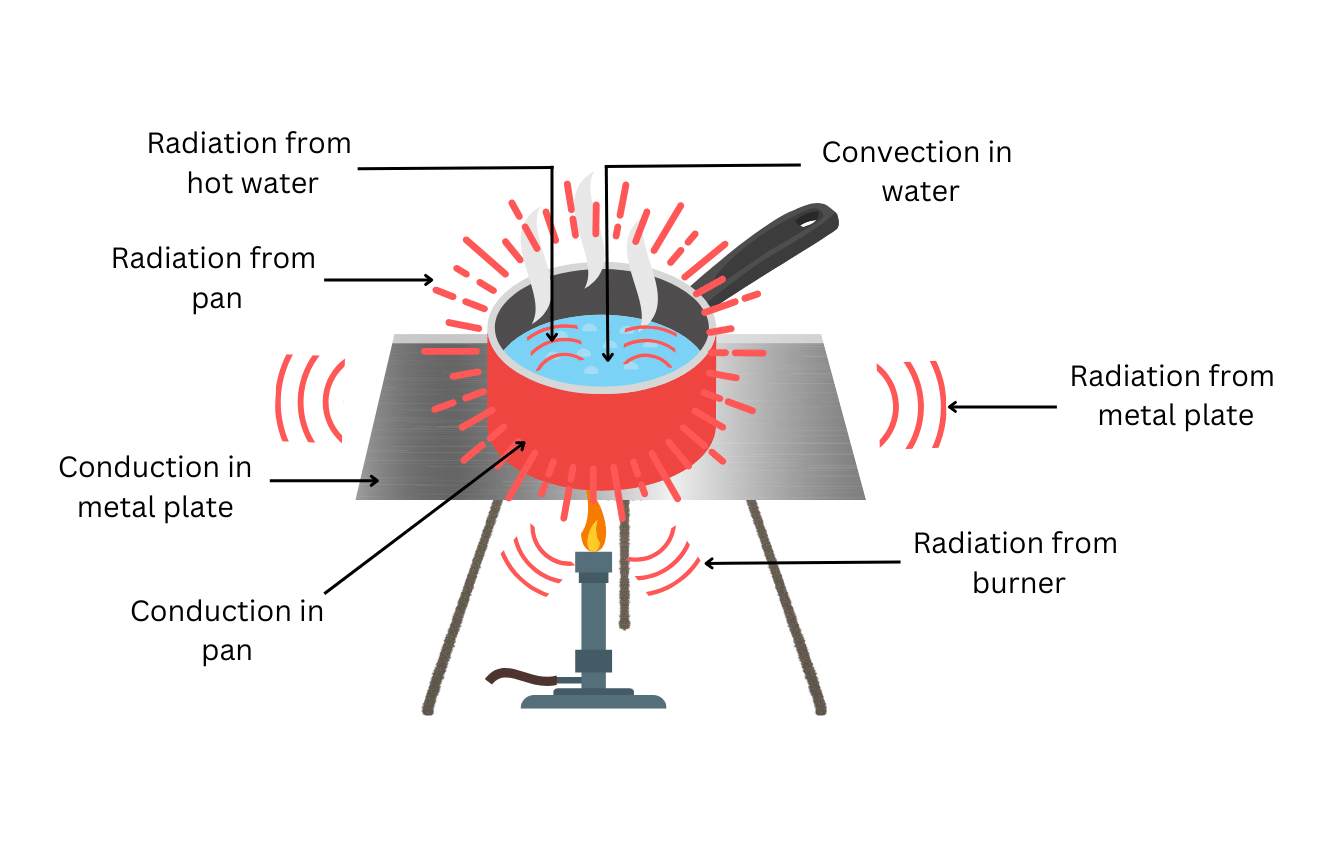
Heat is transferred by the process of conduction in the metal plate and the pan.
Heat is transferred in water by convection.
All hot bodies radiate heat. Therefore, the Bunsen burner, the heated metal plate, the heated pan and the hot water will all radiate heat as shown in the diagram.
Find the completed and labelled diagram below (the three kinds of radiation are marked in red):
7. In places of hot climate it is advised that the outer walls of houses be painted white. Explain.
Answer:
In places of hot climate it is advised that the outer walls of houses be painted white because white is a poor absorber of heat. It reflects most of the heat of the sun that falls on it and this keeps the insides of the house cool.
8. One litre of water at 30°C is mixed with one litre of water at 50°C. The temperature of the mixture will be
(a) 80°C (b) more than 50°C but less than 80°C
(c) 20°C (d) between 30°C and 50°C
Answer: (d) between 30°C and 50°C
When one litre of water at 30°C is mixed with one litre of water at 50°C, the temperature of the mixture will be around 400C. The cooler water at 300 C will gain heat and the hotter water at 500 C will lose heat. The final temperature will be the average of 300 C and 500 C = 400 C.
9. An iron ball at 40°C is dropped in a mug containing water at 40°C. The heat will
(a) flow from the iron ball to water.
(b) not flow from the iron ball to water or from water to the iron ball.
(c) flow from water to the iron ball.
(d) increase the temperature of both.
Answer: (b) not flow from the iron ball to water or from water to the iron ball.
When an iron ball at 40°C is dropped in a mug containing water at 40°C, heat will not flow from the iron ball to water or from water to the iron ball. Here both the temperatures of the iron ball and the water are the same = 400 C. Hence, there will be no heat flow.
10. A wooden spoon is dipped in a cup of ice cream. Its other end
(a) becomes cold by the process of conduction.
(b) becomes cold by the process of convection.
(c) becomes cold by the process of radiation.
(d) does not become cold.
Answer: (d) does not become cold.
If a wooden spoon is dipped in a cup of ice cream its other end does not become cold. Wood is an insulator and a poor conductor of heat. Therefore, when a wooden spoon is dipped in a cup of ice cream, it will not become cold due to conduction.
11. Stainless steel pans are usually provided with copper bottoms. The reason for this could be that
(a) copper bottom makes the pan more durable.
(b) such pans appear colourful.
(c) copper is a better conductor of heat than stainless steel.
(d) copper is easier to clean than stainless steel.
Ans: (c) copper is a better conductor of heat than stainless steel.
Stainless steel pans are usually provided with copper bottoms because copper is a better conductor of heat than stainless steel. Therefore, the stainless steel pans are usually provided with copper bottoms to facilitate good conduction of heat at the point of contact with the flame.
Solutions to Extended Learning – Activities and Projects (Page No 36) of NCERT Class 7 Science Chapter 3 Heat:
1. Got to a doctor or your nearest health centre. Observe the doctor taking the temperature of patients. Enquire:
(a) why she dips the thermometer in a liquid before use.
(b) why the thermometer is kept under the tongue.
(c) whether the body temperature can be measured by keeping the thermometer at some other place than the mouth.
(d) whether the temperature of different parts of the body is the same or different.
Answer:
Solution to Extended Learning – Activities and Projects Problem 1
2. Go to a veterinary doctor (a doctor who treats animals). Discuss and find out the normal temperature of domestic animals and birds:
Answer:
Solution to Extended Learning – Activities and Projects Problem 2
3. Wrap a thin paper strip tightly around an iron rod. Try to burn the paper with candle while rotating the iron rod continuously. Does it burn? Explain your observation.
Answer:
Solution to Extended Learning – Activities and Projects Problem 3
4. Take a sheet of paper. Draw a spiral on it as shown in the Fig. 4.14. Cut out the paper along the line. Suspend the paper as shown in Fig. 4.14 above a lighted candle. Observe what happens. Think of an explanation.
Answer:
Solution to Extended Learning – Activities and Projects Problem 4
5. Take two similar transparent glass bottles having wide mouths. Put a few crystals of potassium permanganate or pour a few drops of ink in one bottle. Fill this bottle with hot water. Fill the other bottle with cold water. Cover the cold water bottle with a thick piece of paper such as a post card. Press the post card firmly with one hand and hold the bottle with the other hand. Invert the bottle and place it on top of the hot water bottle. Hold both the bottles firmly. Ask some other person to pull the post card. Observe what happens. Explain.
Answer:
Solution to Extended Learning – Activities and Projects Problem 5
Solutions to In Text Questions of NCERT Class 7 Science Chapter 3 Heat:
1. (Page 24) In our day-to-day life, we come across a number of objects. Some of them are hot and some of them are cold. Tea is hot and ice is cold. List some objects you use commonly in Table 3.1. Mark these objects as hot or cold
Answer:
The completed table is shown below:
Table 3.1: Hot and Cold objects
| Object | Cold/Cool | Warm/Hot |
| Ice cream | ||
| Spoon in a tea cup | ||
| Fruit juice | ||
| Handle of a frying pan |
2. (Page 25) Boojho says, “My left hand tells me that the water in mug C is hot and the right hand tells me that the same water is cold. What should I conclude?”
Answer:
I conclude that the feelings are relative to each hand. The initial temperature of each hand dictates the feeling of hot or cold when both hands are dipped in mug C. Thus, we see that we cannot always rely on our sense of touch to decide whether an object is hot or cold.
3. (Page 26) Paheli measured her body temperature. She got worried as it was not exactly 37°C.
Answer:
The body temperature of a person can fluctuate based on age, time of day, activity level and individual health conditions. As long as Paheli’s body temperature is around 370 C, she has nothing to worry about.
4. (Page 27) Boojho got a naughty idea. He wanted to measure the temperature of hot milk using a clinical thermometer. Paheli stopped him from doing so.
Answer:
The temperature of hot milk is much greater than the temperature range of a clinical thermometer, which is 350 C – 420 C. The mercury will expand excessively due to the intense heat and exert pressure on the walls of the glass tube. Therefore, the thermometer might break and toxic mercury might spill out.
5. (Page 28) Boojho now understands why clinical thermometer cannot be used to measure high temperatures. But still wonders whether a laboratory thermometer can be used to measure his body temperature.
Answer:
No, a laboratory thermometer cannot be used to measure his body temperature. Unlike in a clinical thermometer,there is nokink to prevent the mercury level from falling in a laboratory thermometer. The mercury level will drop the moment you take the thermometer out of your mouth. This will give you an inaccurate reading.
6. (Page 28) Boojho wonders why the level of mercury should change at all when the bulb of the thermometer is brought in contact with another object?
Answer:
The level of mercury changes when the bulb of the thermometer is brought in contact with another object due to heat flow. If the object is hot, heat flows from the object to the thermometer which causes the mercury in the bulb to expand and rise up the tube.
7. (Page 29) Paheli asks: “Does it mean that heat will not be transferred if the temperature of two objects is the same?”
Answer:
Yes, heat will not be transferred if the temperature of two objects is the same. Heat flows from a hotter body to a colder body. Hence, no heat will be transferred because the temperature of two objects is the same.
Solutions to All Activities of NCERT Class 7 Science Chapter 3 Heat:
1. Complete Activity 3.1 (Page 24). Take three small tubs/containers. Label them as A, B and C. Put cold water in container A and hot water in container B. Mix some cold and hot water in container C. Now dip your left hand in container A and the right hand in container B. After keeping the hands in the two containers for 2–3 minutes, put both the hands simultaneously in container C (Fig. 3.1). Do both the hands get the same feeling?
Answer:
2. Complete Activity 3.2 (Page 25). Let us learn how to read a thermometer. First, note the temperature difference indicated between the two bigger marks. Also note down the number of divisions (shown by smaller marks) between these marks. Suppose the bigger marks read one degree and there are five divisions between them. Then, one small division can read 1/5 = 0.2 °C. Wash the thermometer, preferably with an antiseptic solution. Hold it firmly and give it a few jerks. The jerks will bring the level of mercury down. Ensure that it falls below 35°C. Now place the bulb of the thermometer under your tongue. After one minute, take the thermometer out and note the reading. This is your body temperature. The temperature should always be stated with its unit, °C. What did you record as your body temperature?
Answer:
3. Complete Activity 3.3 (Page 26). Measure the body temperature of some of your friends (at least 10) with a clinical thermometer. Record your observations as in Table 3.2. Is the body temperature of every person 37°C?
Answer:
4. Complete Activity 3.4 (Page 27). Take some tap water in a beaker or a mug. Dip the thermometer in water so that the bulb is immersed in water but does not touch the bottom or the sides of the container. Hold the thermometer vertically (Fig. 3.5). Observe the movement of mercury in the thermometer. Wait till the mercury thread becomes steady. Note the reading.
Answer:
5. Complete Activity 3.5 (Page 28). Take some hot water in a beaker or a mug. Dip the thermometer in water. Wait till the mercury thread becomes steady and note the temperature. Now take out the thermometer from water. Observe carefully what happens now.
Answer:
6. Complete Activity 3.6 (Page 29). Take a rod or flat strip of a metal, say of aluminium or iron. Fix a few small wax pieces on the rod. These pieces should be at nearly equal distances (Fig. 3.7). Clamp the rod to a stand. If you do not find a stand, you can put one end of the rod in between bricks. Now, heat the other end of the rod and observe. What happens to the wax pieces? Do these pieces begin to fall? Which piece falls the first? Do you think that heat is transferred from the end nearest to the flame to the other end?
Answer:
7. Complete Activity 3.7 (Page 30). Heat water in a small pan or a beaker. Collect some articles such as a steel spoon, plastic scale, pencil and divider. Dip one end of each of these articles in hot water (Fig. 3.8). Wait for a few minutes. Touch the other end. Enter your observation in Table 3.3.
Answer:
8. Complete Activity 3.8 (Page 30). Take a round bottom flask (if flask is not available, a beaker can be used). Fill it two-thirds with water. Place it on a tripod, or make some arrangement to place the flask in such a way that you can heat it by placing a candle below it. Wait till the water in the flask is still. Place a crystal of potassium permanganate at the bottom of the flask gently using a straw. Now, heat the water by placing the candle just below the crystal. Write your observation in your notebook and also draw a picture of what you observe.
Answer:
9. Complete Activity 3.9 (Page 31). Light a candle. Keep one hand above the flame and one hand on the side of the flame (Fig. 3.10). Do your hands feel equally hot? If not which hand feels hotter? And why?
Answer:
10. Complete Activity 3.10 (Page No: 32). Take two identical tin cans. Paint the outer surface of one black and of the other white. Pour equal amounts of water in each and leave them in the mid-day sun for about an hour. Measure the temperature of the water in both the cans. Do you find any difference in the temperatures? In which can is the water warmer?
Answer:
11. Complete Activity 3.11 (Page No: 33). Fill the two cans used in Activity 3.10 with the same amount of hot water at the same temperature (say, at 600 C). Leave the cans in a room or in a shade. Note the temperature of water after 10-15 minutes. Does the temperature of water in both the cans fall by the same amount?
Answer:
Extra Questions to Complement Solutions to NCERT Class 7 Science Chapter 3 Heat:
Very Short Answer Type Questions:
1. Name two hot objects.
Answer:
Two hot objects are: the flame of a candle, hot tea.
2. Name two cold objects.
Answer:
Two cold objects are: ice, chilled soft drink.
3. How do we accurately measure the hotness of an object.
Answer:
We can accurately measure the hotness of an object by taking its temperature using a thermometer.
4. Name a substitute for the mercury thermometer.
Answer:
The digital thermometer is a substitute for the mercury thermometer.
5. Where is most of the mercury present in a thermometer before use?
Answer:
Most of the mercury present in a bulb in the thermometer before use.
6. Name a liquid metal.
Answer:
Mercury is a liquid metal.
7. Which scale in a clinical thermometer is used in India?
Answer:
India has adopted the Celsius scale.
8. Do all substances conduct heat easily?
Answer:
No, insulators such as wood, plastic etc cannot conduct heat easily.
9. Give one example where heat travels without any medium.
Answer:
The Sun’s heat travels through vacuum in space via the process of radiation.
10. In which direction does smoke rise?
Answer:
Smoke rises upwards by process of convection because hot air is lighter than cooler air.
11. Why are you advised to use an umbrella when you go out in the sun?
Answer:
You are advised to use an umbrella when you go out in the sun, to protect your skin from being damaged by the UV-radiation of the sun.
Multiple Choice Questions (MCQ):
1. A marble tile would feel cold as compared to a wooden tile on a winter morning, because the marble tile (NCERT Exemplar)
(a) is a better conductor of heat than the wooden tile.
(b) is polished while wooden tile is not polished.
(c) reflects more heat than wooden tile.
(d) is a poor conductor of heat than the wooden tile.
Answer: (a) is a better conductor of heat than the wooden tile.
A marble tile is a better conductor of heat, so the heat escapes from the marble tile to the cold surroundings fast, while in the case of wood it does not.
2. A beggar wrapped himself with a few layers of newspaper on a cold winter night. This helped him to keep himself warm because (NCERT Exemplar)
(a) friction between the layers of newspaper produces heat.
(b) air trapped between the layers of newspaper is a bad conductor of heat.
(c) newspaper is a conductor of heat.
(d) newspaper is at a higher temperature than the temperature of the surrounding.
Answer: (b) air trapped between the layers of newspaper is a bad conductor of heat.
3. Paheli and Boojho measured their body temperature. Paheli found her’s to be 98.6 °F and Boojho recorded 37°C. Which of the following statement is true? (NCERT Exemplar)
(a) Paheli has a higher body temperature than Boojho.
(b) Paheli has a lower body temperature than Boojho.
(c) Both have normal body temperature.
(d) Both are suffering from fever.
Answer: (c) Both have normal body temperature.
98.6 °F is the normal body temperature in the Fahrenheit scale. 370 C is the normal body temperature in the Celsius scale.
Short Answer Type Questions:
1. Why does our hand get burnt when we keep it in contact with a flame, while it does not get burnt if we do not get too close to it?
Answer:
Heat flows from a hot body to a cold body. Direct contact with the flame allows the heat transfer to take place from the flame to our hand. However, if we do not get too close to it, our hand and the flame are separated by air which is a bad conductor of heat. Therefore, the heat transfer does not take place and our hand does not get burnt.
2. Shopkeepers selling ice blocks usually cover them with jute sacks. Explain why.
Answer:
Jute sacks are insulators and cannot conduct heat well. Therefore, if ice is covered with jute sacks, they prevent the heat transfer from the surroundings to the ice (heat flows from hot to cold) and prevent the ice from melting.
3. Why does mercury rise and fall in a thermometer? What prevents it from falling in a clinical thermometer?
Answer:
Liquids like mercury expand on heating and contract when cooled. Therefore, when the heat is transferred from the object to the thermometer, the mercury expands and rises up the tube. When the object is removed the mercury cools down and contracts.
In a clinical thermometer there is a kink which prevents the level of mercury from falling back down when the thermometer is removed. This helps to get accurate readings.
5. If between the two bigger divisions in a clinical thermometer there are 10 divisions. Then what temperature does one small division correspond to?
Answer:
The difference between the two bigger marks corresponds to 10 C. Therefore, if there are 10 divisions between, on small division will correspond to = 0.10 C.
6. Why do have to give the clinical temperature a few extra jerks to bring the mercury level back down after use?
Answer:
The mercury level in a thermometer does not come back down on its own due to the kink. We must bring the mercury level back down after use below the normal body temperature in order to give accurate reading. To give you an example – if the thermometer reads 390 C (before jerking) and your current body temperature is 370 C, if the mercury level is not brought back down you will get a wrong reading.
7. A laboratory thermometer A is kept 7 cm away on the side of the flame while a similar thermometer B is kept 7 cm above the flame of a candle as shown in Figure 4.5. (NCERT Exemplar)
8. To keep her soup warm Paheli wrapped the container in which it was kept with a woollen cloth. Can she apply the same method to keep a glass of cold drink cool? Give reason for your answer.
Answer:
Woollen cloth has layers and pockets in which air gets trapped. Air is an insulator and is a bad conductor of heat. Therefore, a woollen cloth can be used to keep a glass of cold drink cool as it traps air inside which prevents the heat flow from the surroundings to the glass.
9. Why should the thermometer not be held by bulb while reading it?
Answer:
The thermometer should not be held by the bulb because the heat from the hand might cause the mercury in thermometer to further expand, thereby changing the reading.
10. Why should the thermometer be read keeping the level of mercury along the line of sight?
Answer:
The thermometer should be read keeping the mercury level along the line of sight to avoid getting the wrong reading. If the viewing angle is not perpendicular to the position of the eye, you will get a wrong reading as shown in the diagram below:
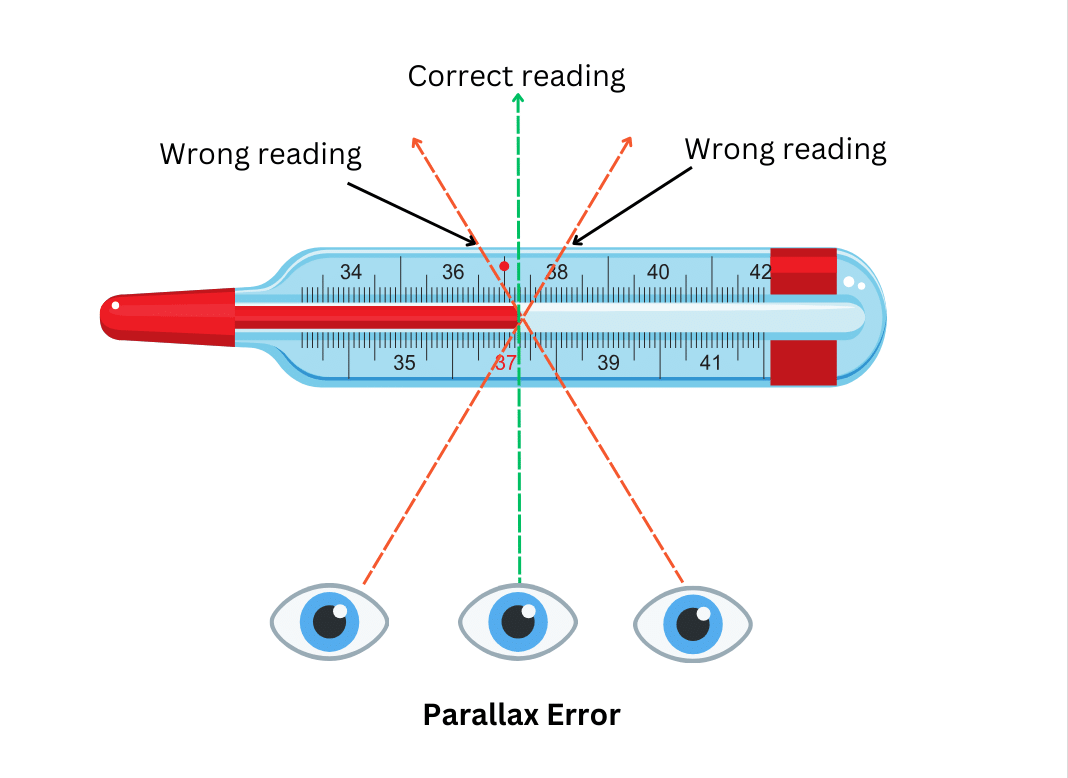
This is called parallax error.
11. Why does our hand feel cold when we touch an ice cube?
Answer:
Our body temperature is higher than an ice cube which is at lower temperature. Heat flows by conduction from a hotter body to colder object. Therefore, the heat from our hand flows to the ice cube, thereby decreasing the temperature of our hand. Therefore, our hand feels cold.
12. How many divisions should a thermometer have?
Answer:
If a thermometer has too few divisions, you will have trouble getting an accurate reading. On the other hand, too many divisions are redundant – you do not need too many divisions to get an accurate reading. Therefore, the number of divisions should not be too few or too many.
13. Give two reasons why water cannot be used instead of mercury in a clinical thermometer.
Answer:
Two reasons why water cannot be used instead of mercury are:
(i) Water is a bad conductor of heat.
(ii) Water will not expand like mercury when in contact with heat.
14. the normal human body temperature always 370 C?
Answer:
The normal human body temperature isnot necessarily = 370 C. The human body temperature fluctuates from time to time and can be slightly higher or slightly lower than 370 C. We say that 370 C is the average human body temperature because that is the average body temperature of a large number of healthy people. However, it may vary from time-to-time and from person-to-person.
15. Why is the range of a clinical thermometer between 350 C and 420 C?
Answer:
The human body temperature normally varies between a low of 350 C and a high of 420 C. It does not usually go beyond that range. Therefore, choosing a range greater than 35 – 420 C would be redundant.
16. What is the bulb of a mercury thermometer made from?
Answer:
The bulb of a mercury thermometer is made of thin-walled glass. Glass is an insulating material and cannot conduct heat well, so the walls of the glass are made thin to help the heat transfer from the body into the mercury inside the bulb.
17. Why is the range of a laboratory thermometer more than that of a clinical thermometer?
Answer:
The range of a laboratory thermometer is greater than that of a clinical thermometer because a laboratory thermometer is used to measure the temperatures of hot liquids. It can also be used to measure the boiling and freezing point of liquids. These temperatures can be quite high (or low if the liquid is very cold) and a greater range is chosen to measure these high temperatures.
18. We know that a laboratory thermometer should not be allowed to touch the bottom of the beaker containing the liquid, which is being heated. Suppose we make a mistake and it touches the bottom. Will the temperature reading in the thermometer greater or lesser than the correct value?
Answer:
The bottom of the flask is the first to receive the heat from the burner and in greatest amount. It takes time for the heat to travel from the bottom of the beaker into the liquid – it does not happen instantly. So, the bottom of the beaker will be hotter than the liquid. Therefore, if the thermometer touches the bottom, the temperature reading in the thermometer will be greater than the temperature of the liquid which we are looking to measure.
19. A pan is heated on a flame and then removed from the fire and cooled. How does heat transfer happen during heating and cooling of the pan?
Answer:
When the pan is being heated, the heat flows from the hotter flame to the cooler pan by conduction. When the pan is removed and cooled, the heat flows from the hotter pan to the colder surroundings by radiation.
20. You must have observed that the metallic pan for cooking has a plastic or wooden handle. Can you lift a hot pan by holding it from the handle without getting hurt?
Answer:
Yes, you can lift a hot pan by the handle without getting hurt. Plastic and wood are both insulators and cannot conduct heat. Therefore, if the handle is made from plastic and wood the heat from the hot metallic pan will not travel to your hand through the handle by conduction and your hand will not get burnt.
21. Give one example of convection in the ocean.
Answer:
Some ocean currents are caused by convection in the ocean. Ocean currents occur when one part of the ocean’s water is heated up more than another part by the Sun. This causes convection currents called ocean currents in which the lighter, warmer water from one part travels to the colder part and the colder water comes in to take its place.
22. Suppose you are given the choice in winter of using either one thick blanket or two thin blankets joined together. What would you choose and why?
Answer:
Two thin blankets would be the better choice. The layer of air trapped between the two blankets would act as an insulator and prevent the heat from our body from flowing into the surroundings. This way our body heat will be maintained and we will feel warm.
23. Can objects with shiny surfaces absorb a large amount of heat?
Answer:
No, the shiny surface will reflect most of the heat that falls on it. Therefore, only a little bit will be absorbed.
24. What happens to heat when it is applied to a body?
Answer:
When heat is applied to a body:
(i) A part of it is reflected.
(ii) A part of it is absorbed by the body.
(iii) A part may be transmitted.
25. How can you construct buildings which are not affected by the heat and cold outside?
Answer:
You can construct the outer walls of buildings with hollow bricks which can trap air inside. Air is an insulator and is a bad conductor of heat. During summer excessive heat will not flow from the surroundings into the buildings – keeping the insides cool. During winter, the insulating air will prevent heat from travelling through the walls into the surroundings – keeping the insides warm.
26. Name two heat-related characteristics of black objects.
Answer:
Black objects are:
(i) Good absorbers of heat.
(ii) Good radiators of heat.
Long Answer Type Questions:
1. Why is mercury used in thermometers?
Answer: Mercury is used in thermometers because:
(i) Mercury expands in response to even a slight increase in temperature. Therefore, it can detect even slight changes in temperatures.
(ii) It expands uniformly.
(iii) It has a high boiling point and can be used for measuring higher temperatures. (iv) It does not stick to the glass surface, therefore the level of mercury can rise and fall easily.
2. In the arrangements A and B shown in the figure, pins P and Q are fixed to a metal loop and an iron rod with the help of wax. In which case are both the pins likely to fall at different times? Explain. (NCERT Exemplar)
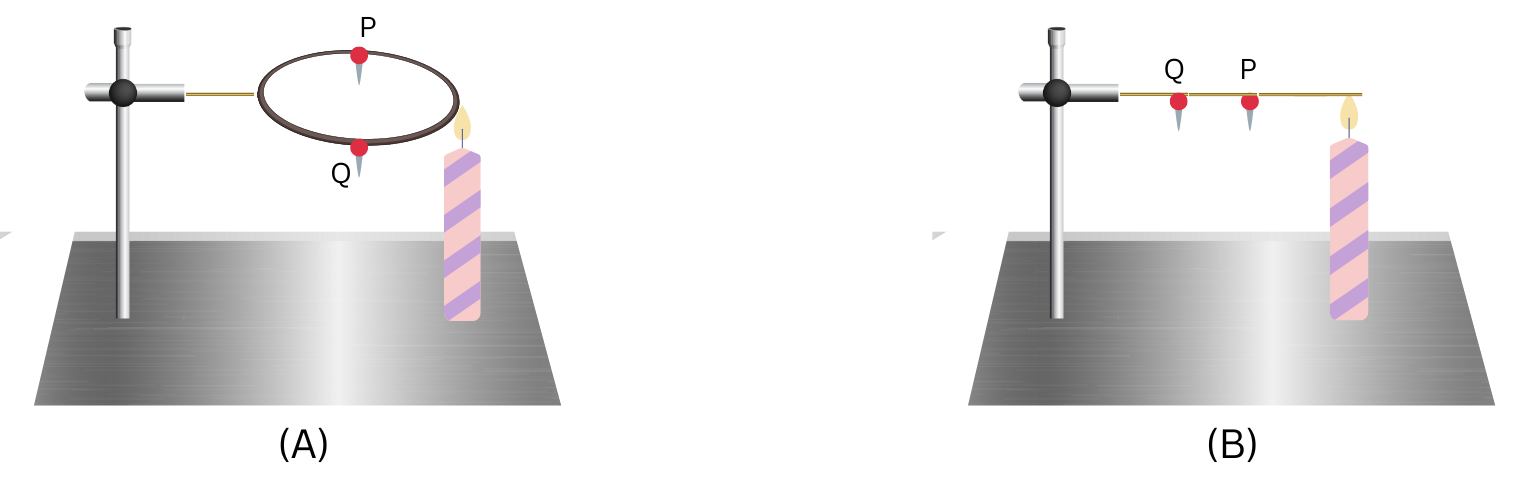
Answer:
For (A): Heat will flow by conduction from the hot flame to the colder metal loop, which is a good conductor of heat (made of metal). The candle is placed equidistant from the points P and Q on the metal loop. The heat will travel the same distance in both directions in the metal loop and the pins P and Q will fall at the same time, after the wax melts due to equal heat in both.
For (B): The heat will flow by conduction from the hot flame to the colder iron rod, which is a good conductor of heat (made of metal). Since the conduction happens from right to left, the pin P will receive heat first and in greater amount than pin Q. Therefore, the pin P will fall first and then pin Q will fall, after the wax melts.
In the arrangement B both the pins will fall at different times.
3. Look at the figure below:
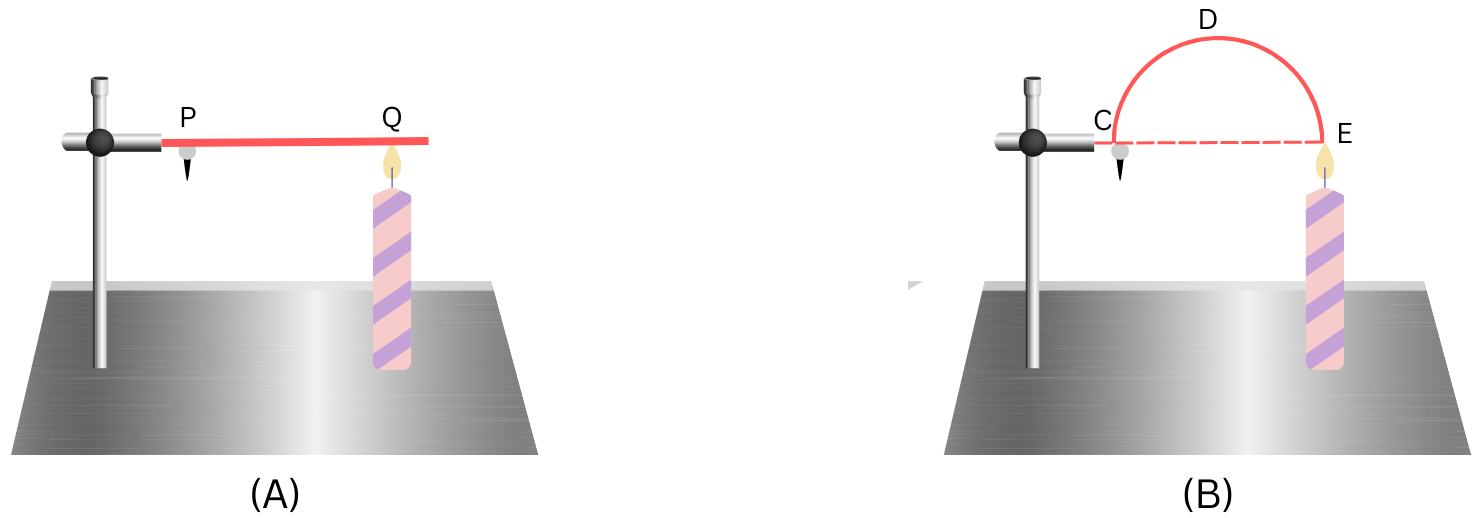
The length of wire PQ in case of A is equal to the diameter of the semicircle formed by the wire CDE, in case B. One pin is attached to each wire with the help of wax as shown in the figure. Which pin will fall first? Explain.
Answer:
The heat will flow by conduction from the hot flame to the colder wire.
We are given that length of PQ = length of diameter CE of semicircular wire CDE.
We know, length of circumference of the semicircle CDE > length of diameter CE.
Therefore, length of circumference of the semicircle CDE > length of PQ.
Therefore, the heat travels faster by conduction from Q to P than it travels through the circumference EDC, starting at the point E.
Therefore, the pin P in (A) receives heat first and in greater amount than pin C in (B). Hence, the pin P will fall first.
4. What are advantages of a digital thermometer over a mercury thermometer?
Answer: The advantages of a digital thermometer over a mercury thermometer are:
(i) It is simple and convenient to use.
(ii) Digital thermometers give instant readings because it does not have to wait for the mercury to rise up.
(iii) It does not contain toxic mercury and is safer to use. If it breaks there is no danger.
(iv) It gives more precise readings than mercury thermometers.
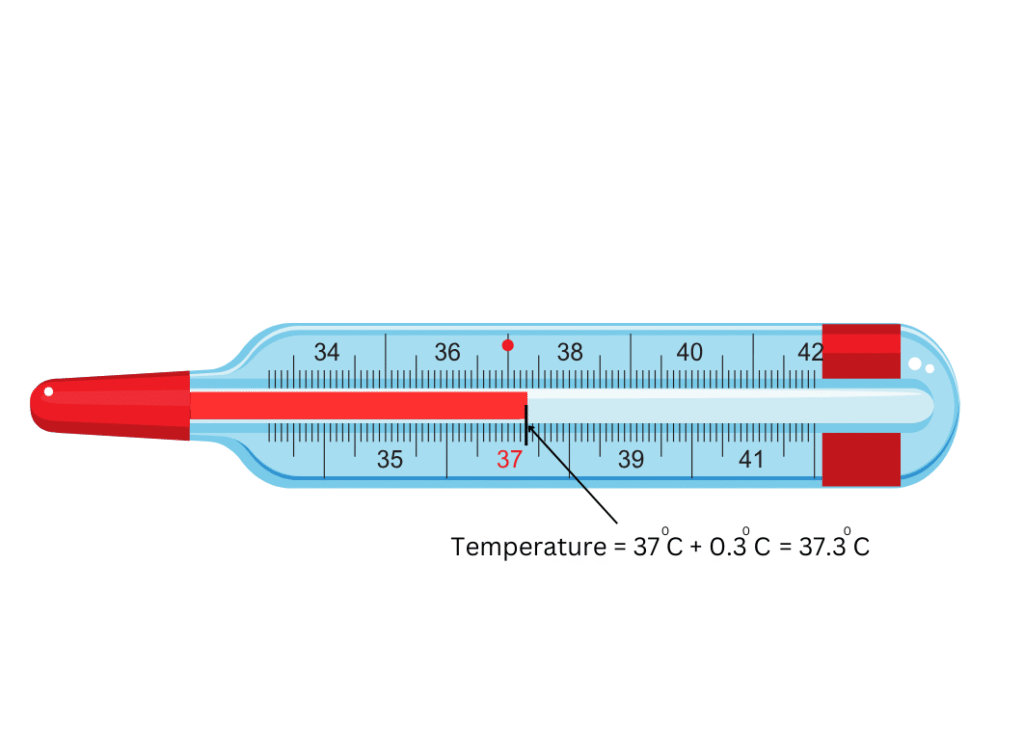
5. Describe how you will read the temperature in the clinical thermometer below:
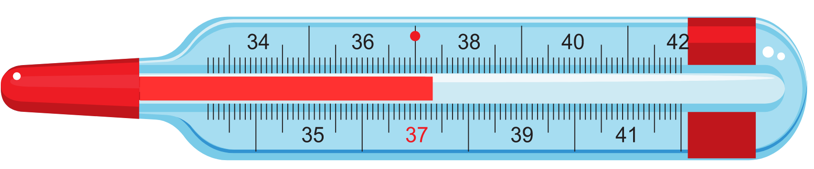
Answer:
The following steps are taken to read the temperature in the thermometer above:
(i) We can see that the temperature difference between the two bigger marks = 10 C.
(ii) Also, by counting we can see that there are 10 divisions between the two bigger marks.
(iii) Therefore, one small division corresponds to = 0.10 C.
(iv) The mercury level is on the third division between 370 C and 380 C.
(v) Therefore, the correct temperature = 370 C + (0.10 × 3) C = 370 C + 0.30 C = 37.30 C. Refer to the figure below for clarity:
6. Explain how a maximum-minimum thermometer works.
Answer:
A maximum-minimum thermometer is used to measure the maximum and minimum temperatures over a certain period of time like for example, an entire day.
It works as follows:
(i) It consists of a U-shaped glass tube with two separate scales along each arm – one for measuring the minimum temperature and one for measuring the maximum temperature.
(ii) There is a sealed glass bulb at the end of each arm. The bulb corresponding to the minimum temperature recording arm is filled with alcohol. The bulb corresponding to the maximum temperature recording arm is filled with vacuum or a low-pressure alcohol vapour. This is shown in red in the figure.
(iii) The U-shaped part of the thermometer is filled with mercury. This is shown in silver in the figure.
(iv) When the external temperature increases, the alcohol in the left tube expands and pushes the mercury down the left tube and up the right tube. The vacuum (or alcohol vapour) in the right tube allows the mercury column to move freely. The maximum temperature will be recorded when there is maximum expansion of alcohol in the left tube, due to which the mercury column reaches the highest point in the right tube on any particular day. This point is marked by a metal index which maintains its position after the mercury column goes back down.
(v) When the external temperature decreases, the alcohol in the left tube contracts and the mercury rises back into the left tube from the right tube. The minimum temperature will correspond to maximum contraction of the alcohol vapour, which allows the mercury column in the left tube to reach its highest point on any particular day. This point is marked by a second metal index which maintains its position after the mercury column goes back down.
(vi) The critical difference is that unlike in a clinical thermometer, in a maximum-minimum thermometer the expansion and contraction of the alcohol column decides the temperature and not the expansion of the mercury column.
Refer to the figure below while reading the explanation for better understanding:
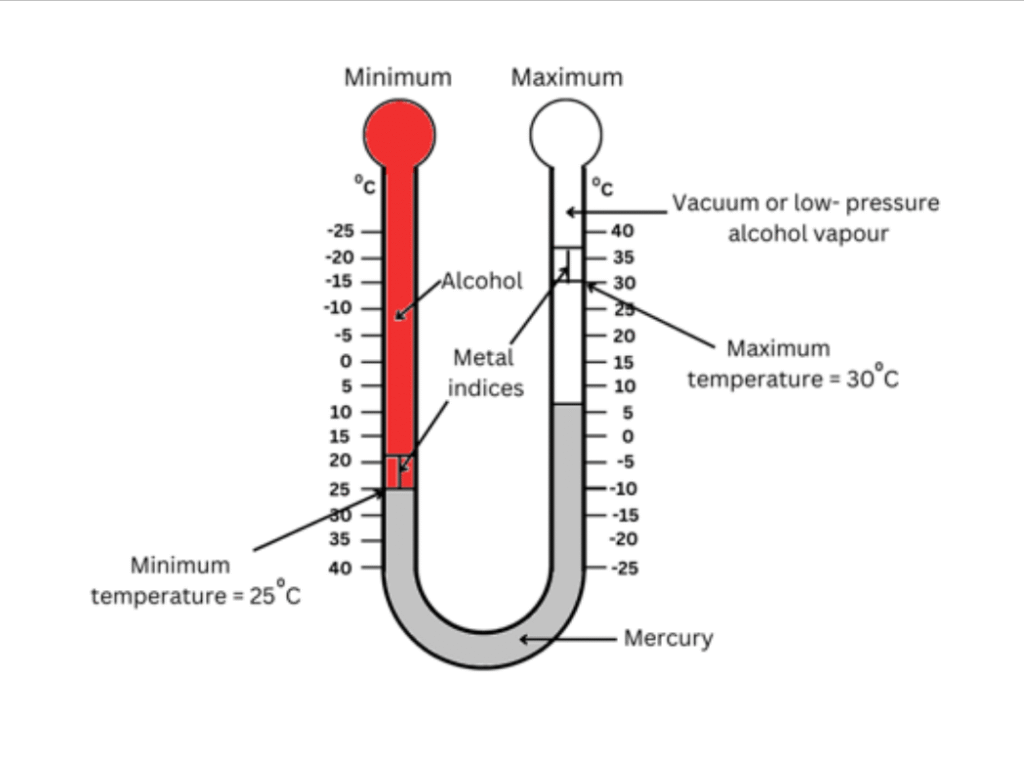
In this case, the maximum temperature = 300 C and minimum temperature = 250 C (as indicated by the indices) for this particular time frame.
7. What is the correct method of using a laboratory thermometer?
Answer:
The correct method of using a laboratory thermometer is described below:
(i) Dip the thermometer into the hot liquid with the bulb of the thermometer immersed in the water.
(ii) Hold it vertically with your hand or with a laboratory clamp, taking care that the thermometer does not touch the bottom or the sides of container.
(iii) If the thermometer touches the bottom or walls of the container, you will get an inaccurate reading as the temperature of the walls of the beaker are different from the liquid.
(iv) Also, the design of the laboratory thermometer is such that you will have to hold it vertically in order to get an accurate reading.
The correct and wrong ways of holding the thermometer are shown in the figures below:

(v) Wait until the mercury thread in the vertically held thermometer rises and becomes steady. Read the temperature of the liquid at that time without taking the thermometer out of the water – if you take the thermometer out of the water, the level of mercury will fall and you will a wrong reading.
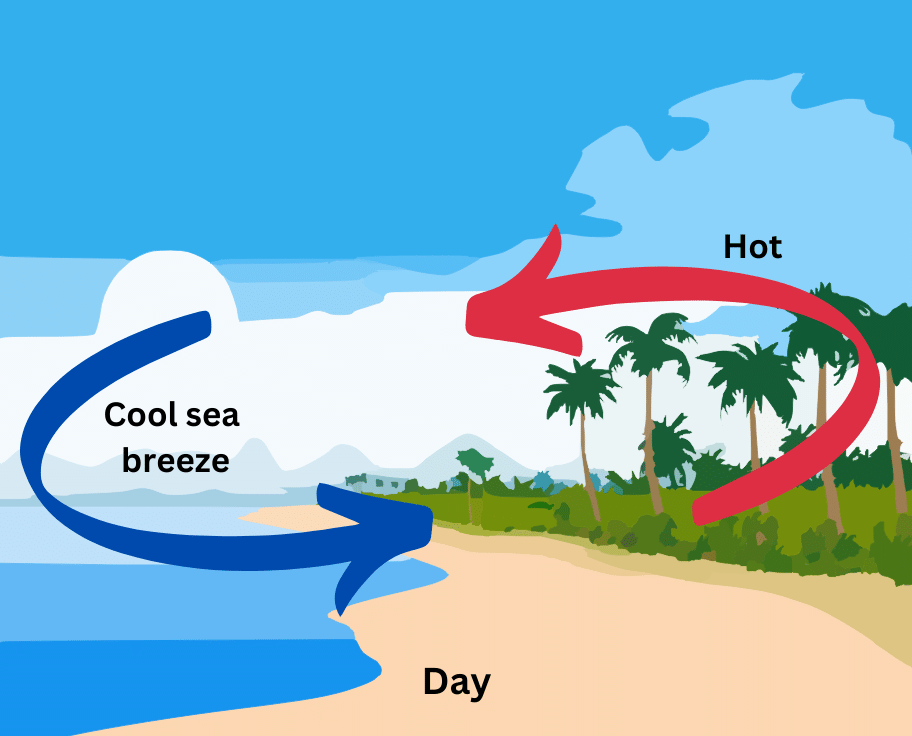
8. Why do coastal areas have moderate climate?
Answer:
Coastal areas have moderate climate due to land and sea breezes:
(i) In coastal areas the land gets heated faster than water during the day. Due to convection the hot air above the land rises and cold air from the sea blows towards the land to take its place. In turn the hot air from the land moves towards the sea to form a cycle. The cold air from the sea is called sea breeze. The formation of the sea breeze, which keeps the coastal area cool during the day is shown below:
(ii) During the night, the cycle is reversed. The water cools down much slower than the land. Therefore, the air over the sea stays warmer than the air over the land, rises up and the cooler air from the land moves towards the sea to take its place. The cooler air from the land is called land breeze. Since the cooler land breeze blows towards the sea and the warmer air from the sea blows towards the land, the coastal area remains warm during the night as shown below:
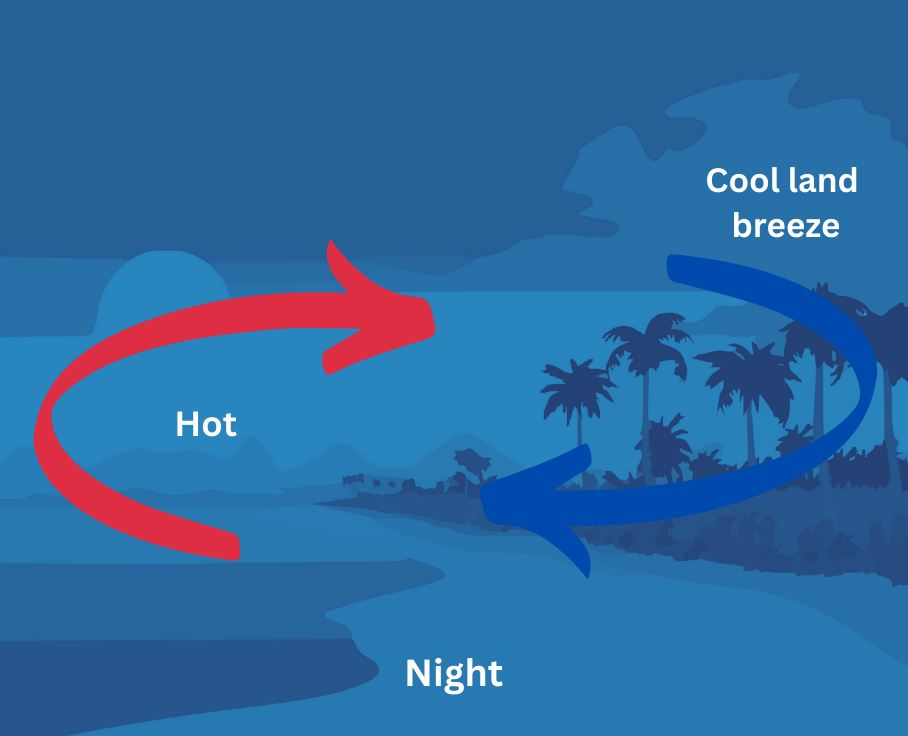
Therefore, we can see that the formation of land and sea breezes maintain moderate climate in the coastal area – neither too hot not too cold.
9. Give some differences between conduction, convection and radiation.
Answer:
The differences between conduction, convection and radiation are:
| Conduction | Convection | Radiation |
| Requires a medium. | Requires a medium. | Does not require a medium. |
| Heat travels via direct contact between two objects. | Heat travels due to actual movement of matter. | Heat travels in the form of waves. |
| Occurs due to temperature difference. | Occur due to difference in density. | Occurs due to heating up of any object. |
| Occurs only in solids. | Occurs in liquids and gases. | Occurs in all bodies. |
| Slow process. | Slow process. | Fast process. |
| Example: When a stove is heated the heat travels to the stove from the flame by conduction. | Example: The water in a stove gets heated by convection. | Example: During heating water the stove gives out heat by radiation. |
Fill in the Blanks:
body temperature, vacuum, reflectors, sea, radiation
(a) During summer months there is more _________.
(b) A digital thermometer is suitable for measuring _________.
(c) While constructing a house in a coastal area, the windows should preferably face towards the _________.
(d) Conduction and convection cannot happen through _________.
(e) Black surfaces are poor _________ of heat.
Answers:
(a) During summer months there is more radiation.
(b) A digital thermometer is suitable for measuring body temperature.
(c) While constructing a house in a coastal area, the windows should preferably face towards the sea.
(d) Conduction and convection cannot happen through vacuum.
(e) Black surfaces are poor reflectors of heat.
Match and Pair:
1. Match the items of Column I with suitable items in Column II:
| Column I | Column II |
| (a) Laboratory thermometer | (i) Radiation |
| (b) Water and air | (ii) Celsius scale |
| (c) Sun | (iii) -100 C – 1100 C |
| (d) Land and sea breezes | (iv) Poor conductors of heat |
| (e) Swedish astronomer | (v) Cool breezes |
Answer:
The correct table is shown below:
| Column I | Column II |
| (a) Laboratory thermometer | (iii) -100 C – 1100 C |
| (b) Water and air | (iv) Poor conductors of heat |
| (c) Sun | (i) Radiation |
| (d) Land and sea breezes | (v) Cool breezes |
| (e) Swedish astronomer | (ii) Celsius scale |
++++++++++++++
Frequently Asked Questions (FAQs) on NCERT Solutions to Class 7 Science Chapter 3 Heat:
These solutions have been prepared by Indian and foreign-educated teachers, engineers and scientists, who have approached the topic from all angles to help you best understand the material. Many practical situations have been shown along with attractive figures, to help you visualize and internalise the concepts. Similar questions will be asked in your exams, so make sure you understand the concepts and problem-solving methods that we have presented here.
The free PDFs of the solutions are available for download anytime! There’s plenty more top-quality study material and resources on the way, which will extremely useful for your preparation. So, make sure you keep visiting our website and subscribe to our email list to be among the first to access them!
The following topics are covered:
3.1 – Hot and Cold
3.2 – Measuring Temperature
3.3 – Laboratory Thermometer
3.4 – Transfer of Heat
3.5 – Kinds of Clothes We Wear in Summer and Winter
Here are the number of problems for the chapter:
(i) 1 Long Answer Type Question (Questions 1)
(ii) 4 Short Answer Type Questions (Questions 2, 5, 7, 8)
(Iii) 1 Fill in the Blanks Question (Question 3)
(iv) 1 Match and Pair Question (Question 4)
(v) 1 Diagram-based Question (Question 6)
(vi) 3 Multiple Choice Questions (Questions 9, 10, 11)
Of course! Feel free to download the free PDF versions of educationroundtheworld.com’s NCERT Solutions for Class 7 Science Chapter 3 Heat for free anytime! All the different types of additional questions are also included in the PDF version for your benefit! Please look towards the top of the page!
All concepts in this chapter are equally important. The best way to master this chapter is to understand the concepts related to heat and then practise answering various types of questions. This way you will gain experience on how to answer many types of questions and your concepts and problem-solving abilities will improve. The concepts in this chapter can be asked in many different ways, so you will have to apply what you have learnt. We have included plenty of conceptual and application-based questions in the extra questions section, which will be an excellent starting point. You will see similar situations and questions in your exams, so learn them well.
We also have expert teacher-mentors on staff who will coach you and give you plenty of practice. Contact us anytime and let us help you out!
We understand that you may face difficulties at any stage of your preparation and may need expert help! Our experienced teacher-mentors understand students’ needs and will coach you and mentor you in the right way to help you succeed in any exam and in your future careers. We provide expert one-on-one coaching and mentoring to you depending on your convenience and needs – have it completely your way! Contact us anytime with your requirements and we would love to help you out! Or simply, book an appointment now!


