Hello students and welcome to Chapter 4 Combustion and Flame! Find excellent solutions to all Chapter 4 exercise questions, extended learning activities, in-text questions and activities for this chapter right here. This is the perfect solutions material you were looking for. All your questions have been thoroughly answered with attractive diagrams wherever necessary. We have also included self-designed extra questions which resemble actual exam questions. Study them in detail and you will be ready for your exams!
Solutions to Exercises (Page No 51) of NCERT Class 8 Science Chapter 4 Combustion and Flame
1. List conditions under which combustion can take place.
Answer:
The conditions under which combustion can take place are:
- Presence of combustible substance i.e. fuel.
- Presence of supporter of combustion i.e. air (oxygen).
- Temperature equal to or higher than ignition temperature is maintained.
2. Fill in the blanks.
(a) Burning of wood and coal causes __________of air.
(b) A liquid fuel, used in homes is__________.
(c) Fuel must be heated to its _________ ________ before it starts burning.
(d) Fire produced by oil cannot be controlled by___________.
Answers:
(a) Burning of wood and coal causes pollution of air.
(b) A liquid fuel, used in homes is kerosene.
(c) Fuel must be heated to its ignition temperature before it starts burning.
(d) Fire produced by oil cannot be controlled by water.
3. Explain how the use of CNG in automobiles has reduced pollution in our cities.
Answer:
The use of CNG in automobiles has reduced pollution in our cities. CNG releases much lesser amounts of carbon dioxide gas, carbon monoxide gas, sulphur dioxide, oxides of nitrogen etc unlike petrol and diesel. Use of CNG also does not result in release of unburnt carbon particles which cause diseases. Thus, CNG is a cleaner fuel.
4. Compare LPG and wood as fuels.
Answer:
LPG is a liquid fuel whereas wood is a solid fuel. LPG has a higher calorific value of 55000 kJ/kg whereas wood has a lower calorific value of 17000 – 22000 kJ/kg. Burning of LPG does not produce smoke whereas burning of wood produces a lot of smoke. Hence, LPG has higher fuel efficiency and is a cleaner fuel.
5. Give reasons.
(a) Water is not used to control fires involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Answers:
(a) Water is not used to control fires involving electrical equipment.
Water is not used to control fires involving electrical equipment because it may conduct electricity and harm those trying to douse the fire.
(b) LPG is a better domestic fuel than wood.
LPG is a better domestic fuel than wood for the following reasons:
- LPG has higher calorific value than wood, so it produces much more heat energy on burning than an equal mass of wood.
- Burning of LPG does not produce smoke whereas burning of wood produces a lot of smoke, which pollutes the atmosphere.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not because aluminium is a good conductor of heat. Aluminium conducts heat away from the paper, which prevents the paper from attaining ignition temperature. Hence, the paper does not burn.
6. Make a labelled diagram of a candle flame.
Answer: The labelled diagram of a candle flame is shown below:
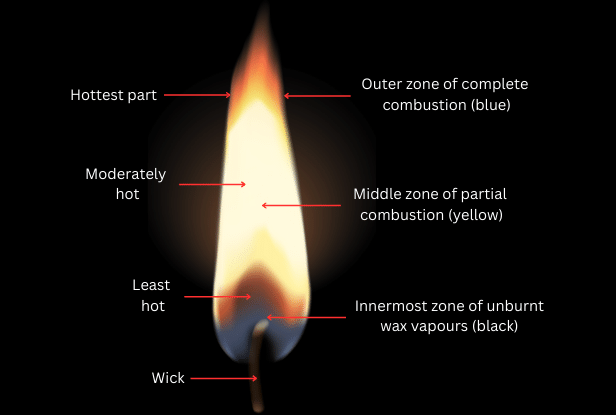
7. Name the unit in which the calorific value of a fuel is expressed.
Answer:
The unit in which the calorific value of a fuel is expressed in kilojoule per kg (kJ/kg).
8. Explain how CO2 is able to control fires.
Answer:
CO2 is able to control fires because it is heavier than oxygen and covers the fire like a blanket. Since the contact between the fuel and oxygen is cut off, the fire is controlled. Also, the added advantage of CO2 is that in most cases it does not harm the electrical equipment.
9. It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Answer:
It is difficult to burn a heap of green leaves but dry leaves catch fire easily because green leaves contain moisture, which increases the ignition temperature. Some of the heat applied is used up by water to evaporate, thus the ignition temperature is not reached. Dry leaves do not contain moisture and ignition temperature is attained easily.
10. Which zone of a flame does a goldsmith use for melting gold and silver and why?
Answer:
The zone of a flame a goldsmith uses for melting gold and silver is the outermost zone or the non-luminous zone. This is because the outermost zone undergoes complete combustion and is the hottest part of the flame. This makes the goldsmith’s job easier.
11. In an experiment 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
Answer:
Calorific value is the amount of heat energy produced on complete combustion of 1 kg of fuel.
When 4.5 kg of the fuel is burnt the heat produced is 180,000 kJ.
Therefore, when 1 kg of the fuel is burnt, the heat produced = 180,000 kJ/4.5 = 40,000 kJ.
Therefore, calorific value = 40,000 kJ/kg.
12. Can the process of rusting be called combustion? Discuss.
Answer:
The process of rusting cannot be called combustion because rusting is not accompanied by release of heat, which is an essential criteria for combustion. Also, moisture is essential for rusting, which is not a condition for combustion.
13. Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water will get heated in a shorter time?
Answer:
Whose water will get heated in a shorter time: Ramesh’s water will get heated in a shorter time. This is because he has kept the beaker in the outermost part of the flame which is hotter and where complete combustion takes place. Abida kept the beaker in the middle zone of partial combustion which is less hot.
Solutions to Extended Learning – Activities and Projects (Page No 52) of NCERT Class 8 Science Chapter 4 Combustion and Flame
1. Survey the availability of various fuels in your locality. Find out their cost per kg and prepare a tabular chart showing how many kJ of various fuels you can get for every rupee.
Answer:
Solution to Extended Learning Problem 1
2. Find out the number, type and location of fire extinguishers available in your school, nearby shops and factories. Write a brief report about the preparedness of these establishments to fight fire.
Answer:
Solution to Extended Learning Problem 2
3. Survey 100 houses in your area. Find the percentage of households using LPG, kerosene, wood and cattle dung as fuel.
Answer:
Solution to Extended Learning Problem 3
4. Talk to people who use LPG at home. Find out what precautions they take in using LPG.
Answer:
Solution to Extended Learning Problem 4
5. Make a model of a fire extinguisher. Place a short candle and a slightly taller candle in a small dish filled with baking soda. Place the dish at the bottom of a large bowl. Light both the candles. Then pour vinegar into the dish of baking soda. Take care. Do not pour vinegar on the candles. Observe the foaming reaction. What happens to the candles? Why? In what order?
Answer:
Solution to Extended Learning Problem 5
Solutions to In Text Questions of NCERT Class 8 Science Chapter 4 Combustion and Flame
1. (Page 40) Can you name a few fuels used in our homes?
Answer:
Natural gas, LPG, kerosene, wood, coal, biofuels.
2. (Page 40) Name a few fuels used in trade and industry.
Answer:
Coal, natural gas, coke, crude oil, diesel, LPG, hydrogen, methane, biofuels.
3. (Page 40) What fuels are used for running automobiles?
Answer:
Petrol, diesel, CNG, LPG, hydrogen, biofuels.
4. (Page 42) We have read that the sun produces its own heat and light. Is it also some kind of combustion?
Answer:
We have read that the sun produces its own heat and light. It is not some kind of combustion because in the sun, heat and light are produced by nuclear reactions.
5. (Page 42) You might have heard that when the clothes of a person catch fire, the person is covered with a blanket to extinguish fire (Fig. 4.3). Can you guess why?
Answer:
The person is covered with a blanket to cut off the oxygen supply to the body. Combustion occurs in the presence of oxygen and so by cutting off the oxygen supply, further burning can be stopped. Thus, the person’s life can be saved.
6. (Page 42) Does a matchstick burn by itself? How does it burn?
Answer:
No a matchstick does not light by itself because its normal temperature is lower than its ignition temperature. Therefore, the matchstick must be rubbed on the side of the matchbox to provide enough heat by friction to surpass the ignition temperature.
7. (Page 42) You must have had an experience of burning a piece of paper. Does it burn when a burning matchstick is brought near it?
Answer:
Yes, paper burns when a burning matchstick is brought near it because of its low ignition temperature.
8. (Page 42) Can you burn a piece of wood by bringing a lighted matchstick near it?
Answer:
You cannot burn a piece of wood by bringing a lighted matchstick near it because the ignition temperature of wood is too high.
9. (Page 42) Why do you have to use paper or kerosene oil to start fire in wood or coal?
Answer:
The ignition temperatures of wood or coal are very high, which makes fires difficult to start. So, we use paper or kerosene to start the fire because these have low ignition temperatures and catch fire immediately. The heat thus generated is enough to reach the ignition temperature of wood.
10. (Page 43) Does it mean that ignition temperature of kerosene oil is lower than that of wood? Does it mean that we need to take special care in storing kerosene oil?
Answer:
Yes it means that the ignition temperature of kerosene oil is lower than that of wood. The reason is that it only takes kerosene oil a little bit of heat to start a fire, whereas wood takes a lot more heat to start burning. Therefore, we need to take special care in storing kerosene oil to avoid accidents.
11. (Page 46) Observe an LPG flame. Can you tell the colour of the flame? What is the colour of a candle flame?
Answer:
The colour of LPG flame is bluish. The colour of a candle flame is yellowish.
12. (Page 46) Recall your experience of burning a magnesium ribbon in Class VII. If you do not have experience of burning the remaining items in Table 4.2 you can do that now.
Answer:
The completed Table 4.2 is shown below:
| S. No. | Material | Forms flame | Does not form flame |
| 1. | Candle | Yes | |
| 2. | Magnesium | Yes | |
| 3. | Camphor | Yes | |
| 4. | Kerosene stove | Yes | |
| 5. | Charcoal | Yes |
13. (Page 48) Goldsmiths blow the outermost zone of a flame with a metallic blow-pipe for melting gold and silver (Fig. 4.14). Why do they use the outermost zone of the flame?
Answer:
Goldsmiths blow the outermost zone of a flame because it is the hottest part of the flame where complete combustion takes place. This high temperature is crucial for efficiently melting gold and silver, which have high melting points.
14. (Page 48) Make a list of fuels familiar to you. Group them as solid, liquid and gaseous fuels as in Table 4.3.
Answer:
The completed Table 4.2 is shown below:
| S. No. | Solid Fuels | Liquid Fuels | Gaseous Fuels |
| 1. | Coal | Kerosene oil | Natural gas |
| 2. | Wood | Petrol | LPG |
| 3. | Charcoal | Diesel | Hydrogen |
| 4. | Coke | Crude oil | Methane |
15. (Page 48) Suppose you were asked to boil a given quantity of water using cow dung, coal and LPG as fuel. Which fuel would you prefer? Give your reason. You may take the help of your parents. Do these three fuels produce the same amount of heat?
Answer:
I would prefer LPG as fuel because it is a clean fuel and releases lesser amounts of pollutants than cow dung and coal. Also, its calorific value is the highest, which indicates that it produces the highest heat energy for a given mass.
Solutions to All Activities of NCERT Class 8 Science Chapter 4 Combustion and Flame
1. Complete Activity 4.1 (Page 41). Collect some materials like straw, matchsticks, kerosene oil, paper, iron nails, stone pieces, glass etc. Under the supervision of your teacher try to burn each of these materials one by one. If combustion takes place mark the material combustible, otherwise mark it non-combustible (Table 4.1). Can you name some more substances which are combustible? You can add those to Table 4.1.
Table 4.1: Combustible and Non-combustible Substances
| Material | Combustible | Non-combustible |
| Wood | ||
| Paper | ||
| Iron nails | ||
| Kerosene oil | ||
| Stone piece | ||
| Straw | ||
| Charcoal | ||
| Matchsticks | ||
| Glass |
Answer:
2. Complete Activity 4.2 (Page 41). (Caution: Be careful while handling burning candle). Fix a lighted candle on a table. Put a glass chimney over the candle and rest it on a few wooden blocks in such a way that air can enter the chimney [Fig. 4.2(a)]. Observe what happens to the flame. Now remove the blocks and let the chimney rest on the table [Fig. 4.2(b)]. Again observe the flame. Finally, put a glass plate over the chimney [Fig. 4.2(c)]. Watch the flame again. What happens in the three cases? Does the flame flicker off? Does it flicker and give smoke? Does it burn unaffected? Can you infer anything at all about the role played by air in the process of burning?
Answer:
3. Complete Activity 4.3 (Page 42). Place a piece of burning wood or charcoal on an iron plate or Tawa. Cover it with a glass jar or a tumbler, or a transparent plastic jar. Observe what happens. Does charcoal stop burning after sometime? Can you think of the reason why it stops burning?
Answer:
4. Complete Activity 4.4 (Page 43). (Caution: Be careful while handling burning candle). Make two paper cups by folding a sheet of paper. Pour about 50 mL of water in one of the cups. Heat both the cups separately with a candle (Fig. 4.5). What do you observe?
Answer:
5. Complete Activity 4.5 (Page 47). Light a candle (Caution : Be careful). Hold a 4-5 cm long thin glass tube with a pair of tongs and introduce its one end in the dark zone of a non-flickering candle flame (Fig. 4.10). Bring a lighted matchstick near the other end of the glass tube. Do you see a flame caught at this end of the glass tube after a while? If so, what is it that produces a flame?
Answer:
Extra Questions to Complement Solutions to NCERT Class 8 Science Chapter 4 Combustion and Flame
Very Short Answer Type:
1. Name a solid fuel used in rural areas for cooking?
Answer:
Cow dung.
2. What is a substance which undergoes combustion called?
Answer:
Fuel.
3. Which materials burns in air with a brilliant white light?
Answer:
Magnesium.
4. Name a dangerous substance which was used in matchsticks earlier.
Answer:
White phosphorus.
5. Name an easily combustible substance present in a car.
Answer:
Petrol.
6. Name a clean fuel that can be used in cars instead of petrol.
Answer:
Compressed Natural Gas (CNG).
7. What does the amount of heat energy produced on combustion of a given substance depend on?
Answer:
Calorific value of the substance.
8. Name the unit of heat.
Answer:
Joule.
9. Give an example of spontaneous combustion that occurs in nature.
Answer:
Forest fires.
10. Why is the interior of the candle flame dark?
Answer:
Due to presence of unburnt carbon particles.
Multiple Choice Questions (MCQ):
1. A substance that undergoes spontaneous combustion is:
(a) LPG
(b) Phosphorus
(c) Cooking oil
(d) Wood
Answer: (b) Phosphorus
Explanation: Phosphorus burns in air at room temperature, hence it fits the criteria of spontaneous combustion.
2. Which fuel has the highest fuel efficiency:
(a) Coal
(b) Wood
(c) Hydrogen
(d) LPG
Answer: (c) Hydrogen
3. Which zone of the candle has unburnt wax vapours?
(a) Outer
(b) Middle
(c) Innermost
(d) All of the above
Answer: (c) Innermost
4. Which of the following fuels releases a lot of smoke when burnt:
(a) LPG
(b) Wood
(c) CNG
(d) Hydrogen
Answer: (b) Wood
5. Name the harmful gas released by incomplete combustion of fuels.
(a) Carbon dioxide
(b) Carbon monoxide
(c) Sulphur dioxide
(d) Nitrogen dioxide
Answer: (b) Carbon monoxide
Short Answer Type:
1. Food is a fuel for our body. Explain.
Answer:
Food is broken down by reaction with oxygen in our body and heat is produced. Hence, food is a fuel.
2. Why are there forest fires during the summer?
Answer:
During extreme heat of summer, dry grass catches fire because its ignition temperature is low. From grasses, the fire spreads to trees and soon the whole forest gets set on fire.
3. Do all materials produce a flame on burning? Explain.
Answer:
No, only the substances which vapourise on burning produce flames. Materials such as charcoal do not produce vapours on burning and hence does not produce a flame.
4. Why should kerosene not be kept near a stove?
Answer:
Kerosene has low ignition temperature and catches fire very easily. Therefore, it could lead to a serious accident.
5. How does water work to extinguish a fire?
Answer:
Water cools down the combustible material and brings its temperature below ignition temperature. Water vapour also surrounds the combustible material and cuts off the supply of air. These two factors work together to extinguish a fire.
6. Suppose a petrol pump catches on fire. Should you water to extinguish the fire?
Answer:
No, because water is denser than petrol and will sink below the layer of petrol. Thus, the petrol will continue to burn on top of the water and the fire will not be extinguished.
7. Suppose a petrol pump catches on fire. What should you use to control the fire?
Answer:
You should use carbon dioxide. Carbon dioxide being heavier than oxygen covers the fire like a blanket. Since contact between fuel and oxygen is cut off, the fire is controlled.
8. Give the characteristics of an ideal fuel.
Answer:
The characteristics of a good fuel are that it is cheap, readily available, readily combustible, produces a large amount of heat, easy to transport and does not leave behind harmful substances on burning.
9. What are the harmful products released by burning of fuels like wood, coal and petroleum?
Answer:
Harmful carbon particles, carbon monoxide gas, carbon dioxide, oxides of sulphur and nitrogen are released by burning of fuels like wood, coal and petroleum.
10. What are the consequences of global warming?
Answer:
Global warming increases the temperature of the atmosphere of the earth. This results in melting of polar glaciers, which leads to the rise in the sea level and subsequent flooding in coastal areas. The long-term effects include coastal areas becoming permanently submerged under water.
Long Answer Type:
1. Describe a modern safety match.
Answer:
A modern safety match is described as follows:
(i) The head of the safety match contains only antimony trisulphide and potassium chlorate.
(ii) The rubbing surface has powdered glass and a little red phosphorus (which is much less dangerous than white phosphorus).
(iii) When the match is struck against the rubbing surface, red phosphorus gets converted into white phosphorus.
(iv) This immediately reacts with potassium chlorate in the matchstick head to produce enough heat to ignite antimony trisulphide and start the reaction.
2. (a) Look at the images below of the glass plate against the candle. Can you identify the zone of the flame in which the glass plate is held?
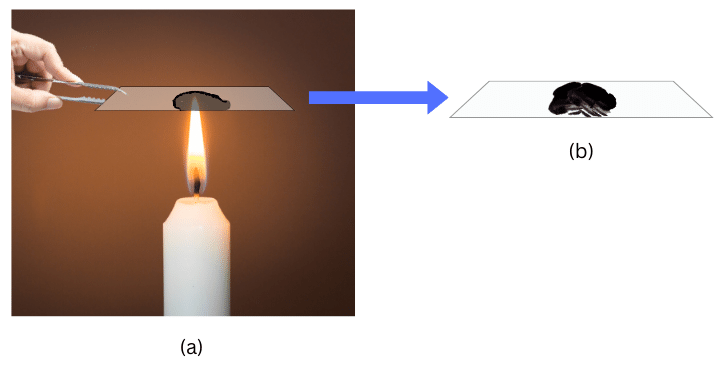
(b) Look at the image of the copper wire that is held against the flame for just 30 seconds. Which part of the flame is it held against?

Answers:
(a) The glass plate is held against the luminous zone of the flame. We conclude this from the circular blackish ring comprising of unburnt carbon particles. These particles are only present in the luminous zone of the flame.
(b) The portion of the copper just outside the flame gets red hot after just 30 seconds. That indicates that the copper wire is held against the non-luminous or the hottest part of the flame.
Fill in the Blanks:
heat, blue, light, Egypt, low, carbon dioxide
(a) Coal burns in air producing __________, __________ and __________.
(b) Matches were first invented in ancient _________.
(c) Inflammable substances have very _________ ignition temperature.
(d) An LPG stove burns with a _________ flame.
(e) ________ does not form vapours on burning.
Answers:
(a) Coal burns in air producing carbon dioxide, heat and light.
(b) Matches were first invented in ancient Egypt.
(c) Inflammable substances have very low ignition temperature.
(d) An LPG stove burns with a blue flame.
(e) Charcoal does not form vapours on burning.
Match and Pair:
| Column A | Column B |
| (i) Combustion | (a) Non-luminous |
| (ii) Outer zone of candle flame | (b) Low calorific value |
| (iii) Coal dust | (c) Chemical change |
| (iv) Oxide of sulphur | (d) Acid rain |
| (v) Cow dung | (e) Spontaneous combustion |
Answer:
The correct table is shown below:
| Column A | Column B |
| (i) Combustion | (c) Chemical change |
| (ii) Outer zone of candle flame | (a) Non-luminous |
| (iii) Coal dust | (e) Spontaneous combustion |
| (iv) Oxide of sulphur | (d) Acid rain |
| (v) Cow dung | (b) Low calorific value |
++++++++++++++
Frequently Asked Questions (FAQs) on NCERT Solutions to Class 8 Science Chapter 4 Combustion and Flame
Our expert team of engineers and scientists have prepared these solutions, taking care to answer every question that you might have about this chapter. All Chapter 4 exercise questions, extended learning activities, in-text questions, in-text activities and even self-designed extra questions have been thoroughly answered in this chapter. If you study these solutions in detail, you will get a complete understanding of the topic and will be able to answer exam questions well. Attractive diagrams have been included wherever necessary and will further help in understanding the material.
The free PDFs of the solutions are available for download anytime! Do you like our material? Then please subscribe to our email list to be the among the first to receive fresh study material and resources!
The following topics are covered:
4.1 – What is Combustion?
4.2 – How Do We Control Fire?
4.3 – Types of Combustion
4.4 – Flame
4.5 – Structure of Flame
4.6 – What is a Fuel?
4.7 – Fuel Efficiency
Here are the number of problems for the chapter:
(i) 10 Short Answer Type Questions (Questions 1, 3, 4, 7, 8, 9, 10, 11, 12, 13)
(ii) 1 Fill in the Blanks Type Question (Question 2)
(iii) 1 Long Answer Type Question (Question 5)
(iv) 1 Diagram-based Question (Question 6)
Yes indeed! Feel free to download the PDF versions of educationroundtheworld.com’s NCERT Solutions for Class 8 Science Chapter 4 Combustion and Flame anytime you please! The entire material is included in the PDF version. Please look towards the top of the page to find the download button!
Study the activities in this chapter well, they will help you understand the concepts related to combustion. Other important parts of the chapter are the parts on how to extinguish fires and fuel efficiency. We have explained the activities and the rest of the chapter with attractive diagrams and explanations, so study our material and the diagrams well. They will help improve your concepts.
Do you need us to coach you from scratch? Our personable teacher-mentors will be there to teach you anytime you need them. Feel free to contact us anytime and let us help you out. Book an appointment now!


