Hello students and welcome to Class 9! Find here the complete solutions material for Chapter 1 where we have answered all in-text questions, exercise questions, group activity and in-text activities. We have also included a set of extra questions for your benefit. The solutions have designed with extreme accuracy, keeping in mind the students’ and NCERT’s requirements. Attractive figures have been included wherever necessary and will help you visualise the concepts perfectly!
Solutions to In Text Questions of NCERT Class 9 Science Chapter 1 Matter in Our Surroundings –
Page 3:
1. Which of the following are matter?
Chair, air, love, smell, hate, almonds, thought, cold, lemon water, smell of perfume.
Answer:
The following substances are matter: chair, air, smell, almonds, lemon water, smell of perfume because they occupy space and have mass. Smell is considered matter because particles of the substance we are smelling mix with particles of air and reach our nose. These particles occupy space and have mass.
2. Give reasons for the following observation: The smell of hot sizzling food reaches you several metres away, but to get the smell from cold food you have to go close.
Answer:
The smell of hot sizzling food reaches you several metres away because high temperature provides food particles with high kinetic energy and so they diffuse through the air quickly. In contrast, to get the smell from cold food you have to go close because they have low kinetic energy and cannot diffuse quickly.
3. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Answer:
A diver is able to cut through water in a swimming pool and the property of matter this observation shows is that there is enough space between particles of water. The strength of the forces acting between the particles in water is not very high and thus there is enough space between them.
4. What are the characteristics of the particles of matter?
Answer:
The characteristics of the particles of matter are:
- Particles of matter have space between them.
- Particles of matter are constantly moving.
- Particles of matter attract each other.
- Particles of matter are very small.
Page 6:
1. The mass per unit volume of a substance is called density. (density = mass/volume). Arrange the following in order of increasing density – air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Answer:
The substances arranged in increasing order of density are: air < exhaust from chimneys < cotton < water < honey < chalk < iron.
2. (a) Tabulate the differences in the characteristics of states of matter.
(b) Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Answer:
(a) The differences in the characteristics of states of matter are tabulated below:
| Characteristics | Solid | Liquid | Gas |
| Shape | Fixed shape | No fixed shape | No fixed shape |
| Volume | Fixed volume | Fixed volume | No fixed volume |
| Force between particles | High | Less than solids | Less than liquids |
| Space between particles | Low | Greater than solids | Greater than liquids |
| Rigidity/Fluidity | Rigid with distinct boundaries, cannot flow | Not rigid, can flow. | Not rigid, flows even more than liquids. |
| Boundary | Definite boundary | No definite boundary | No definite boundary |
| Compressibility | Negligible | Compressible | Highly compressible |
| Kinetic energy | Minimum | Intermediate | Maximum |
(b) Rigidity: Rigidity is the tendency of a substance to maintain its shape under application of a force. Solids are rigid, while liquids and gases are not.
Compressibility: When an external force results in the particles of a substance to come closer, thereby reducing the distance between them, then the substance is said to have high compressibility. Solids have negligible compressibility while liquids and gases are highly compressible.
Fluidity: Fluidity is property of a substance which enables it to flow or move about freely. Solids are rigid and not fluid, while liquids and gases can flow.
Filling a gas container: Gases have high kinetic energy and can flow easily, therefore they can easily fill a gas container. Gases have high compressibility, so a large amount of gas can be filled into one container.
Shape: Solids have definite shape, while liquids and gases have no definite shape and take the shape of the container.
Kinetic energy and density: Kinetic energy is the energy of an object because of its motion. Solids have minimum, liquids have intermediate and gases have high kinetic energy.
Density is the mass per unit volume of substance. Solids have highest density, followed by liquids and then gases.
3. Give reasons
(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
Answers:
(a) A gas fills completely the vessel in which it is kept.
A gas fills completely the vessel in which it is kept because gas particles have weak force of attraction between them. Therefore, they are free to move about and fill the vessel completely.
(b) A gas exerts pressure on the walls of the container.
A gas exerts pressure on the walls of the container because gas particles are in constant motion with high kinetic energy and hit the walls of the container at high speed.
(c) A wooden table should be called a solid.
A wooden table should be called a solid because it has a fixed shape and volume with rigid boundaries and cannot flow. The force between particles is high and space between particles is low. It is also not compressible.
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
We can easily move our hand in air because air particles have very low force of attraction between them and are far apart from each other. But to do the same through a solid block of wood we need a karate expert because the solid wood has high force of attraction between particles and they are close together.
4. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Answer:
Although liquids have lower density as compared to solids, ice has lower density than water. When water freezes into ice, the particles are farther apart than in the liquid state. This arrangement causes ice to be less dense than liquid water. Hence, it is observed that ice floats on water.
Page 9:
1. Convert the following temperature to celsius scale:
a. 300 K b. 573 K
Answer:
a. 273 K = 0oC
300 K = (300 – 273) = 27oC.
b. 273 K = 0oC
573 K = (573 – 273) = 300oC.
2. What is the physical state of water at:
a. 250oC b. 100oC ?
Answers:
a. 250oC
The physical state of water at 250oC is gaseous state. This is because the boiling point of water is 100oC and 250oC is much above that.
b. 100oC
The physical state of water at 100oC is both liquid and gaseous because 100oC is the boiling point of water.
3. For any substance, why does the temperature remain constant during the change of state?
Answer:
During a change of state, such as melting or boiling, the temperature remains constant. This is because the energy being absorbed or released is used to overcome or form attraction forces between particles rather than increasing the kinetic energy of the particles.
4. Suggest a method to liquefy atmospheric gases.
Answer:
One method to liquefy atmospheric gases is to increase the pressure. The gas can be taken in a cylinder and pressure can be exerted using a piston. The gas particles will come close together and the forces of attraction will turn the gas into a liquid.
Page 10:
1. Why does a desert cooler cool better on a hot dry day?
Answer:
A desert cooler cools better on a hot dry day because of high temperature and low humidity, both of which enhance evaporation. The increased rate of evaporation of water cools down the surroundings more.
2. How does the water kept in an earthen pot (matka) become cool during summer?
Answer:
Water kept in an earthen pot (matka) becomes cool during summer because of evaporation of water through the pores of the vessel. The energy lost during evaporation is replaced by the energy from the surrounding water, which makes it cool.
3. Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Answer:
Our palm feels cold when we put some acetone or petrol or perfume on it because they gain energy from our palm to evaporate. This causes the palm to feel cold.
4. Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
Answer:
We are able to sip hot tea or milk faster from a saucer rather than a cup because the saucer has a larger surface area which enhances evaporation. The increased rate of evaporation cools down the tea or milk faster and we can drink it safely.
5. What type of clothes should we wear in summer?
Answer:
We should wear cotton clothes in the summer for easy absorption of sweat. This exposes the sweat to the atmosphere for easy evaporation. The heat energy required for evaporation is absorbed from the body, leaving the body cool.
Solutions to Exercises (Page No 12) of NCERT Class 9 Science Chapter 1 Matter in Our Surroundings –
1. Convert the following temperatures to the celsius scale.
(a) 293 K (b) 470 K
Answer:
(a) 293 K
273 K = 0oC
293 K = (293 – 273) = 20oC.
(b) 470 K
273 K = 0oC
470 K = (470 – 273) = 197oC.
2. Convert the following temperatures to the kelvin scale.
(a) 25°C (b) 373°C
Answer:
(a) 25°C
0oC = 273 K
25°C = (25 + 273) K = 298 K.
(b) 373°C
0oC = 273 K
373°C = (373 + 273) K = 646 K.
3. Give reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
Answers:
(a) Naphthalene balls disappear with time without leaving any solid.
Naphthalene balls disappear with time without leaving any solid because they undergo sublimation directly from solid to gaseous state. Eventually all the solid naphthalene changes into gas.
(b) We can get the smell of perfume sitting several metres away.
We can get the smell of perfume sitting several metres away because in the gaseous state, the perfume particles have high speed and large spaces between them. This allows the perfume particles to diffuse very fast into air particles and reach our noses quickly and at a distance.
4. Arrange the following substances in increasing order of forces of attraction between the particles— water, sugar, oxygen.
Answer:
Water is liquid, sugar is solid and oxygen is gas. Therefore, the substances arranged in increasing order of forces of attraction between the particles are: oxygen < water < sugar.
5. What is the physical state of water at—
(a) 25°C (b) 0°C (c) 100°C ?
Answers:
(a) 25°C
The physical state of water at 25oC is liquid because 25oC is room temperature.
(b) 0°C
The physical state of water at 0°C is both solid and liquid because 0°C is freezing point of water.
(c) 100°C
The physical state of water at 100°C is both liquid and gas because 100°C is boiling point of water.
6. Give two reasons to justify—
(a) water at room temperature is a liquid.
(b) an iron almirah is a solid at room temperature.
Answers:
(a) water at room temperature is a liquid.
Water at room temperature is a liquid because:
- Forces of attraction between water particles are less.
- Spaces between particles and kinetic energy of particles are more.
(b) an iron almirah is a solid at room temperature.
An iron almirah is a solid at room temperature because:
- Forces of attraction between iron particles are very large.
- Spaces between iron particles and kinetic energy of particles are very small.
7. Why is ice at 273 K more effective in cooling than water at the same temperature?
Answer:
Ice at 273 K absorbs heat energy, specifically latent heat, from the surroundings to overcome the fusion process and transition into water. In contrast, water does not absorb this extra latent heat and is not as effective at cooling. For this reason, ice at 273 K is more effective in cooling than water at the same temperature.
8. What produces more severe burns, boiling water or steam?
Answer:
Steam produces more severe burns than boiling water because steam contains more energy. This is because when water gets converted to steam at 1000C, the latent heat of vapourisation is added to the steam in addition to the energy of boiling water. Thus steam contains more energy and causes more severe burns.
9. Name A,B,C,D,E and F in the following diagram showing change in its state

Answer:
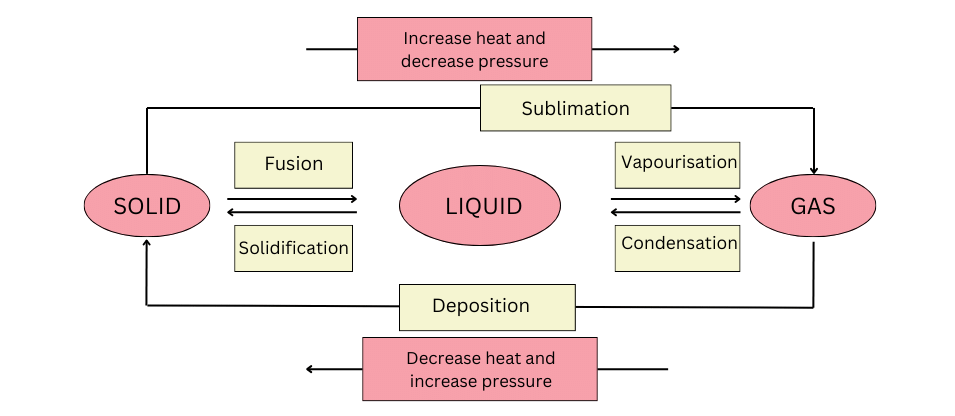
Find the correct labels below:
(A) Change of solid to liquid – Fusion
(B) Change of liquid to gas – Vapourisation
(C) Change of gas to liquid – Condensation
(D) Change of liquid to solid – Solidification
(E) Change of solid to gas – Sublimation. Increasing temperature and decreasing pressure can change a solid to gas. This is called sublimation.
(F) Change of gas to solid – Deposition. Decreasing temperature and increasing pressure can change a gas to solid. This is called deposition.
Solution to Group Activity (Page No 13) of NCERT Class 9 Science Chapter 1 Matter in Our Surroundings –
Prepare a model to demonstrate movement of particles in solids, liquids and gases.
For making this model you will need
• A transparent jar
• A big rubber balloon or piece of stretchable rubber sheet
• A string
• Few chickpeas or black gram or dry green peas.
How to make?
• Put the seeds in the jar.
• Sew the string to the centre of the rubber sheet and put some tape to keep it tied securely.
• Stretch and tie the rubber sheet on the mouth of the jar.
• Your model is ready. Now run your fingers up and down the string by first tugging at it slowly and then rapidly.
Answer:
Solutions to All Activities of NCERT Class 9 Science Chapter 1 Matter in Our Surroundings –
1. Complete Activity 1.1 (Page 1).
• Take a 100 mL beaker.
• Fill half the beaker with water and mark the level of water.
• Dissolve some salt/ sugar with the help of a glass rod.
• Observe any change in water level.
• What do you think has happened to the salt?
• Where does it disappear?
• Does the level of water change?
Answer:
2. Complete Activity 1.2 (Page 1).
• Take 2–3 crystals of potassium permanganate and dissolve them in 100 mL of water.
• Take out approximately 10 mL of this solution and put it into 90 mL of clear water.
• Take out 10 mL of this solution and put it into another 90 mL of clear water.
• Keep diluting the solution like this 5 to 8 times.
• Is the water still coloured?
Answer:
3. Complete Activity 1.3 (Page 2).
• Put an unlit incense stick in a corner of your class. How close do you have to go near it so as to get its smell?
• Now light the incense stick. What happens? Do you get the smell sitting at a distance?
• Record your observations.
Answer:
4. Complete Activity 1.4 (Page 2).
• Take two glasses/beakers filled with water.
• Put a drop of blue or red ink slowly and carefully along the sides of the first beaker and honey in the same way in the second beaker.
• Leave them undisturbed in your house or in a corner of the class.
• Record your observations.
• What do you observe immediately after adding the ink drop?
• What do you observe immediately after adding a drop of honey? • How many hours or days does it take for the colour of ink to spread evenly throughout the water?
Answer:
5. Complete Activity 1.5 (Page 2).
• Drop a crystal of copper sulphate or potassium permanganate into a glass of hot water and another containing cold water. Do not stir the solution. Allow the crystals to settle at the bottom.
• What do you observe just above the solid crystal in the glass?
• What happens as time passes?
• What does this suggest about the particles of solid and liquid?
• Does the rate of mixing change with temperature? Why and how?
Answer:
6. Complete Activity 1.6 (Page 3).
• Play this game in the field— make four groups and form human chains as suggested:
• The first group should hold each other from the back and lock arms like Idu-Mishmi dancers (Fig. 1.3).
• The second group should hold hands to form a human chain.
• The third group should form a chain by touching each other with only their finger tips.
• Now, the fourth group of students should run around and try to break the three human chains one by one into as many small groups as possible.
• Which group was the easiest to break? Why?
• If we consider each student as a particle of matter, then in which group the particles held each other with the maximum force?
Answer:
7. Complete Activity 1.7 (Page 3).
• Take an iron nail, a piece of chalk and a rubber band.
• Try breaking them by hammering, cutting or stretching. • In which of the above three substances do you think the particles are held together with greater force?
Answer:
8. Complete Activity 1.8 (Page 3).
• Take some water in a container, try cutting the surface of water with your fingers.
• Were you able to cut the surface of water?
• What could be the reason behind the surface of water remaining together?
Answer:
9. Complete Activity 1.9 (Page 4).
• Collect the following articles — a pen, a book, a needle and a piece of wooden stick.
• Sketch the shape of the above articles in your notebook by moving a pencil around them.
• Do all these have a definite shape, distinct boundaries and a fixed volume?
• What happens if they are hammered, pulled or dropped?
• Are these capable of diffusing into each other?
• Try compressing them by applying force. Are you able to compress them?
Answer:
10. Complete Activity 1.10 (Page 4).
• Collect the following:
(a) water, cooking oil, milk, juice, a cold drink.
(b) containers of different shapes. Put a 50 mL mark on these containers using a measuring cylinder from the laboratory.
• What will happen if these liquids are spilt on the floor?
• Measure 50 mL of any one liquid and transfer it into different containers one by one. Does the volume remain the same?
• Does the shape of the liquid remain the same ?
• When you pour the liquid from one container into another, does it flow easily?
Answer:
11. Complete Activity 1.11 (Page 5).
• Take three 100 mL syringes and close their nozzles by rubber corks, as shown in Fig.1.4.
• Remove the pistons from all the syringes.
• Leaving one syringe untouched, fill water in the second and pieces of chalk in the third.
• Insert the pistons back into the syringes. You may apply some vaseline on the pistons before inserting them into the syringes for their smooth movement.
• Now, try to compress the content by pushing the piston in each syringe.
• What do you observe? In which case was the piston easily pushed in?
• What do you infer from your observations?
Answer:
12. Complete Activity 1.12 (Page 6).
• Take about 150 g of ice in a beaker and suspend a laboratory thermometer so that its bulb is in contact with the ice, as in Fig. 1.6.
• Start heating the beaker on a low flame.
• Note the temperature when the ice starts melting.
• Note the temperature when all the ice has converted into water.
• Record your observations for this conversion of solid to liquid state.
• Now, put a glass rod in the beaker and heat while stirring till the water starts boiling.
• Keep a careful eye on the thermometer reading till most of the water has vaporised.
• Record your observations for the conversion of water in the liquid state to the gaseous state.
Answer:
13. Complete Activity 1.13 (Page 8).
• Take some camphor or ammonium chloride. Crush it and put it in a china dish.
• Put an inverted funnel over the china dish.
• Put a cotton plug on the stem of the funnel, as shown in Fig. 1.7.
• Now, heat slowly and observe.
• What do you infer from the above activity?
Answer:
14. Complete Activity 1.14 (Page 9).
• Take 5 mL of water in a test tube and keep it near a window or under a fan.
• Take 5 mL of water in an open china dish and keep it near a window or under a fan.
• Take 5 mL of water in an open china dish and keep it inside a cupboard or on a shelf in your class.
• Record the room temperature.
• Record the time or days taken for the evaporation process in the above cases.
• Repeat the above three steps of activity on a rainy day and record your observations. • What do you infer about the effect of temperature, surface area and wind velocity (speed) on evaporation?
Answer:
Extra Questions to Complement Solutions to NCERT Class 9 Science Chapter 1 Matter in Our Surroundings –
Very Short Answer Type Questions:
1. Name the three states of matter.
Answer:
Solid, liquid and gas.
2. Name the SI unit of mass.
Answer:
Kilogram (kg).
3. Name the SI unit of volume.
Answer:
Cubic metre (m3).
4. Is matter continuous or particulate?
Answer:
Matter is particulate.
5. Out of the three states of matter which has the most kinetic energy?
Answer:
Gas.
6. Name the process by which water slowly turns into vapour on its own.
Answer:
Evaporation.
7. Evaporation happens faster in the summer than in winter. Which factor is responsible for this?
Answer:
Temperature.
8. Give an example of a substance which undergoes sublimation.
Answer:
Camphor.
9. Large volumes of gas can be compressed into a small cylinder. Which property of gas is this?
Answer:
Compressibility.
10. Name the state of matter which consists of super energetic and super excited particles.
Answer:
Plasma.
Multiple Choice Questions (MCQ):
1. Name the unit of pressure.
(A) Atmosphere
(B) Pascal
(C) Kilogram
(D) Newton
Answer: (B) Pascal
2. The conversion of water vapour to liquid water is known as:
(A) Condensation
(B) Solidification
(C) Vapourisation
(D) Deposition
Answer: (A) Condensation
3. The state of matter formed by cooling a gas of extremely low density to super low temperatures is known as:
(A) Solid
(B) Plasma
(C) Bose-Einstein Condensate
(D) Vapour
Answer: (C) Bose-Einstein Condensate
4. In which of the following conditions, the distance between the molecules of hydrogen gas would increase? (NCERT Exemplar)
(i) Increasing pressure on hydrogen contained in a closed container
(ii) Some hydrogen gas leaking out of the container
(iii) Increasing the volume of the container of hydrogen gas
(iv) Adding more hydrogen gas to the container without increasing the volume of the container
(a) (i) and (iii)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer: (c) (ii) and (iii)
Increasing the volume of the container would mean that the same number of particles now occupy more space. So, the distance between the molecules would increase. If some hydrogen leaked out, then there would be lesser number of particles inside the container. Therefore, the distance between the molecules would increase.
5. The arrangement of particles is least ordered in case of:
(A) Solids
(B) Liquids
(C) Gases
(D) All of the above
Answer: (C) Gases
Short Answer Type Questions:
1. When we dissolve salt in water, why does the level of water not change?
Answer:
The level of water does not change because the salt is made up of tiny particles which get into the spaces between the particles of water. This does not affect the overall level.
2. A plastic ruler and a wooden ruler are given to you. Which is harder to break and why?
Answer:
The wooden ruler would be harder to break because the forces of attraction between the particles of wood are greater than the forces of attraction between the plastic particles. Hence, it takes more force to overcome the forces of attraction between the wood particles and this makes it harder to break.
3. Why does water not suddenly turn into vapour on its own?
Answer:
The particles of water are held together by forces of attraction between them. The particles do not have sufficient kinetic energy to overcome the forces of attraction and turn into vapour.
4. Why does higher temperature favour evaporation?
Answer:
Higher temperature provides more particles with more kinetic energy. Due to this reason, these particles have enough energy to overcome the forces of attraction in the liquid state and escape to form vapour.
5. A rubber band stretches on application of force. Is it a solid?
Answer:
We know that solids are rigid and do not change shape easily. Even though the rubber stretches, it is still a solid because it regains its shape when the force is removed.
6. Alka was making tea in a kettle. Suddenly she felt intense heat from the puff of steam gushing out of the spout of the kettle. She wondered whether the temperature of the steam was higher than that of the water boiling in the kettle. Comment. (NCERT Exemplar)
Answer:
Yes, the temperature of the steam was higher than that of the water boiling in the kettle. This is because the particles of steam contain extra energy in the form of latent heat.
7. Why does the temperature of water remain constant during melting of ice to water?
Answer:
During melting of ice to water the heat energy is used to overcome the forces of attraction between particles of ice and is not used to heat the water. This continues until all the ice has converted into water. Thus, the temperature of water remains constant.
8. Why is solid carbon dioxide known as dry ice?
Answer:
Solid carbon dioxide gas gets converted directly into gaseous state on decrease of pressure to 1 atmosphere without first turning into liquid state. Hence, it is known as dry ice.
9. If you fan yourself the sweat on your body evaporates faster. why is that?
Answer:
Fanning yourself introduces movement and circulation of air over the surface of your skin, which carries away the water vapor molecules produced by sweat. Therefore, the capacity for evaporation improves and the sweat evaporates faster.
10. Define latent heat of vapourisation.
Answer:
Latent heat of vapourisation is the heat energy required to change 1 kg of a liquid to gas at atmospheric pressure at its boiling point.
11. When you mix lemonade with water diffusion is faster than when a potassium permanganate crystal is place in water. Explain.
Answer:
The rate of diffusion in liquids like lemonade is higher than in solids because in the liquid state particles move freely and have greater interparticular space as compared to solids.
Long Answer Type Questions:
1. Study the graph for melting of ice at 0oC until it forms vapour. Identify the different states of matter marked with A, B, C and D in the graph.
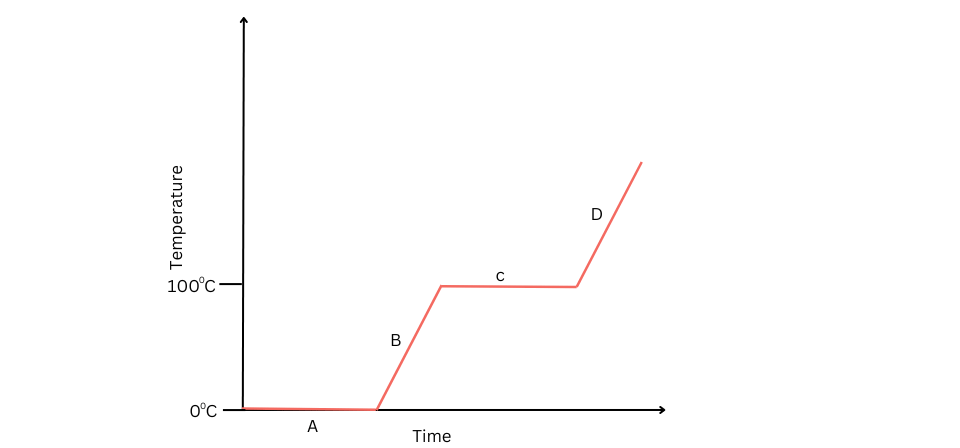
Answer:
A – Ice and water
B – Water
C – Water and vapour
D – Vapour
In A, ice is melting into liquid. Therefore, both ice and water are present. Temperature remains constant at 0o C.
In B, all the ice has long since melted into water and water is being heated up.
In C, water is boiling. Therefore, both water and vapour are present. Temperature remains constant at 100oC.
In D, all the water has long since boiled off and converted into vapour and the vapour is being heated.
Fill in the Blanks:
(a) 1 L = _______ dm3.
(b) The intermixing of particles of two different types of matter is called __________.
(c) Solids have _________ compressibility.
(d) A substance liquefies under application of pressure. It is a _________.
(e) All forms of matter have _________ and _________.
Answers:
(a) 1 L = 1 dm3.
(b) The intermixing of particles of two different types of matter is called diffusion.
(c) Solids have negligible compressibility.
(d) A substance liquefies under application of pressure. It is a gas.
(e) All forms of matter have mass and volume.
Match and Pair:
| Column A | Column B |
| (i) Solids | (a) Diffusion |
| (ii) Oxygen dissolved in water | (b) Solid and liquids |
| (iii) Latent heat | (c) Rigid |
| (iv) Fixed volume | (d) Compressed gas |
| (v) LPG | (e) Hidden heat |
Answer:
| Column A | Column B |
| (i) Solids | (c) Rigid |
| (ii) Oxygen dissolved in water | (a) Diffusion |
| (iii) Latent heat | (e) Hidden heat |
| (iv) Fixed volume | (b) Solid and liquids |
| (v) LPG | (d) Compressed gas |
++++++++++++++
Frequently Asked Questions (FAQs) on NCERT Solutions to Class 9 Science Chapter 1 Matter in Our Surroundings –
Our expert team of Indian and foreign-educated engineers and scientists have answered all in-text questions, exercise questions, group activity and in-text activities in this material, making it a complete solutions package. They have included a set of extra questions, which will further clear your concepts and prepare you for your exams.
The free PDFs of the solutions are also available for download anytime! Want more top-quality material from us? Keep visiting our website and subscribe to our email list to be among the first to access them.
The following topics are covered:
1.1 Physical Nature of Matter
1.2 Characteristics of Particles of Matter
1.3 States of Matter
1.4 Can Matter Change its State?
1.5 Evaporation
Here are the number of problems for the chapter:
(i) 8 Short Answer Type Questions (Questions 1 – 8)
(ii) 1 Long Answer Type Question (Questions 9)
Yes indeed! You can download the PDF versions of educationroundtheworld.com’s NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings anytime you please! We have included the entire material in the PDF version for your benefit! Please look towards the top of the page to find the download button!
There are a lot of theoretical concepts in this chapter which are very fundamental and which you will need to understand deeply. You will see a lot of theoretical questions from this chapter, so make sure you learn it well and practise using the concepts. Practise answering the extra questions we have included for this chapter, to test your preparation.
If you need extra coaching, let us help you! We have expert teachers on staff who love coaching and mentoring bright young students like you. So, feel free to reach out to us anytime and let us help you out!
Need counselling regarding your future academic and professional careers? Our experts believe in mentoring a student regarding their future, in addition to coaching them for exams. This ‘teacher-mentor’ approach boosts students’ confidence and improves their performance in school as well. If you like what we offer, please feel free to reach out to us anytime. We provide expert one-on-one coaching and mentoring to you depending on your convenience and needs – have it completely your way! Book live online classes now!


