Hello students! Chapter 3 Atoms and Molecules is a very basic chapter and it is essential that you grasp these concepts from the beginning. In this complete solutions material, we have answered all in-text questions, exercise questions, group activity and in-text activities for your benefit. All your questions have been answered thoroughly and we have also supplied you with a set of extra questions to give you additional practice.
Solutions to In Text Questions of NCERT Class 9 Science Chapter 3 Atoms and Molecules
Page 27:
1. In a reaction, 5.3 g of sodium carbonate reacted with 6 g of acetic acid. The products were 2.2 g of carbon dioxide, 0.9 g water and 8.2 g of sodium acetate. Show that these observations are in agreement with the law of conservation of mass.
sodium carbonate + acetic acid → sodium acetate + carbon dioxide + water
Answer:
Law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction, i.e. mass remains the same.
In the given reaction,
Mass of reactants = 5.3 g + 6 g = 11.3 g.
Mass of products = 2.2 g + 0.9 g + 8.2 g = 11.3 g.
Therefore, mass of reactants = mass of products and this is in agreement with the law of conservation of mass.
2. Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water. What mass of oxygen gas would be required to react completely with 3 g of hydrogen gas?
Answer:
Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water.
For 1 g of hydrogen, the amount of oxygen required = 8 g.
For 3 g of hydrogen, the amount of oxygen required = 3 × 8 = 24 g.
Therefore, mass of oxygen gas that would be required to react completely with 3 g of hydrogen gas = 24 g.
3. Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass?
Answer:
The postulate of Dalton’s atomic theory which is the result of the law of conservation of mass is, “Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.”
4. Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
Answer:
The postulate of Dalton’s atomic theory that can explain the law of definite proportions is, “The relative number and kinds of atoms are constant in a given compound.”
Page 30:
1. Define the atomic mass unit.
Answer:
One atomic mass unit is a mass unit equal to exactly one-twelfth (1/12th) the mass of one atom of carbon-12. The relative atomic masses of all elements have been found with respect to an atom of carbon-12.
2. Why is it not possible to see an atom with naked eyes?
Answer:
It is not possible to see an atom with naked eyes because atoms are too small. Atomic radius is measured in nanometres. Also, an atom cannot exist independently, they can only exist as molecules or ions.
Page 34:
1. Write down the formulae of
(i) sodium oxide
(ii) aluminium chloride
(iii) sodium suphide
(iv) magnesium hydroxide
Answer:
The formulae are:
(i) sodium oxide – Na2O
(ii) aluminium chloride – AlCl3
(iii) sodium sulphide – Na2S
(iv) magnesium hydroxide – Mg (OH)2
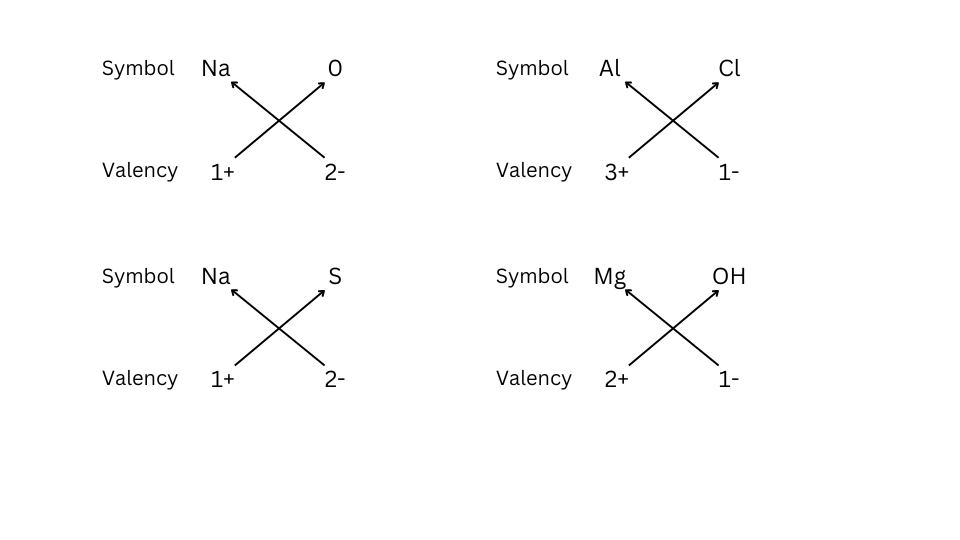
2. Write down the names of compounds represented by the following formulae:
(i) Al2(SO4)3 (ii) CaCl2 (iii) K2SO4 (iv) KNO3 (v) CaCO3
Answer:
Below are the names of compounds represented by the following formulae:
(i) Al2(SO4)3 – Aluminium sulphate
(ii) CaCl2 – Calcium chloride
(iii) K2SO4 – Potassium sulphate
(iv) KNO3 – Potassium nitrate
(v) CaCO3 – Calcium carbonate
3. What is meant by the term chemical formula?
Answer:
The chemical formula of a compound is a symbolic representation of its composition. It tells us the number and types of different atoms present in the compound. For example, the formula of hydrogen chloride is HCl. We can tell from the formula that the number of hydrogen atoms is 1 and the number of chlorine atoms is also 1.
4. How many atoms are present in a
(i) H2S molecule and
(ii) PO43– ion?
Answer:
(i) H2S molecule has 3 atoms present (2 atoms of hydrogen and 1 atom of sulphur).
(ii) PO43– ion has 5 atoms present (1 atom of phosphorus and 4 atoms of oxygen)
Page 35:
1. Calculate the molecular masses of H2, O2, Cl2, CO2, CH4, C2H6, C2H4, NH3, CH3OH.
Answer:
Molecular mass of H2 = 2 × atomic mass of H = 2 × 1u = 2u.
Molecular mass of O2 = 2 × atomic mass of O = 2 × 16u = 32u.
Molecular mass of Cl2 = 2 × atomic mass of Cl = 2 × 35.5u = 70u.
Molecular mass of CO2 = atomic mass of C + 2 x atomic mass of O = 12 + (2 × 16) u = 44u.
Molecular mass of CH4 = atomic mass of C + 4 x atomic mass of H = 12 + (4 × 1) u = 16u.
Molecular mass of C2H6 = 2 × atomic mass of C + 6 x atomic mass of H = 2 × 12 + (6 × 1) u = 30u.
Molecular mass of C2H4 = 2 × atomic mass of C + 4 x atomic mass of H = 2 × 12 + (4 × 1) u = 28u.
Molecular mass of NH3 = Atomic mass of N + 3 x atomic mass of H = 14 + 3 × 1 u = 17 u.
Molecular mass of CH3OH = atomic mass of C + 3 x atomic mass of H + atomic mass of O + atomic mass of H = (12 + 3 × 1 + 16 + 1)u = (12 + 3 + 17)u = 32u.
2. Calculate the formula unit masses of ZnO, Na2O, K2CO3, given atomic masses of Zn = 65u,
Na = 23u, K = 39u, C = 12u, and O = 16u.
Answer:
Atomic mass of Zn = 65u
Atomic mass of Na = 23u
Atomic mass of K = 39u
Atomic mass of C = 12u
Atomic mass of O = 16u
Therefore,
The formula unit mass of ZnO = Atomic mass of Zn + Atomic mass of O = 65u + 16u = 81u.
The formula unit mass of Na2O = 2 x Atomic mass of Na + Atomic mass of O = (2 x 23)u + 16u = 46u + 16u = 62u.
The formula unit mass of K2CO3 = 2 x Atomic mass of K + Atomic mass of C + 3 x Atomic mass of O = (2 x 39)u + 12u + (3 x 16)u = 78u + 12u + 48u = 138u.
Summary: The formula unit masses of ZnO, Na2O, K2CO3 are 81u, 62u and 138u respectively.
Solutions to Exercises (Page No 36) of NCERT Class 9 Science Chapter 3 Atoms and Molecules
1. A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by weight.
Answer:
Mass of compound = 0.24 g.
Mass of boron = 0.096 g.
Mass of oxygen = 0.144 g.
Percentage of boron = (Mass of boron)/(Mass of compound) × 100 = 0.096/0.24 × 100 = 40%.
Percentage of oxygen = (100 – 40)% = 60%.
Therefore, the compound contains 40% boron and 60% oxygen.
2. When 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of oxygen? Which law of chemical combination will govern your answer?
Answer:
We are given that when 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced.
The law of constant proportions states that, “In a chemical substance the elements are always present in definite proportions by mass.”
This means the compound will always have the elements in the same proportions by mass, irrespective of how the compound was prepared.
Therefore, even if the amount of oxygen is increased to 50 g, 3.0 g of carbon will again burn in 8.00 g oxygen to produce 11 g of carbon dioxide.
(50 – 8) g = 42 g of oxygen remains unreacted.
The law of chemical combination governing the answer is the law of constant proportions.
3. What are polyatomic ions? Give examples.
Answer:
Polyatomic ions are groups of atoms carrying a charge and behave as a single unit. Some examples are OH–, CO32-, SO42-.
4. Write the chemical formulae of the following.
(a) Magnesium chloride
(b) Calcium oxide
(c) Copper nitrate
(d) Aluminium chloride
(e) Calcium carbonate.
Answer:
The chemical formulae of the following are:
(a) Magnesium chloride – MgCl2
(b) Calcium oxide – CaO
(c) Copper nitrate – Cu(NO3)2
(d) Aluminium chloride – AlCl3
(e) Calcium carbonate – CaCO3
5. Give the names of the elements present in the following compounds.
(a) Quick lime
(b) Hydrogen bromide
(c) Baking powder
(d) Potassium sulphate.
Answer:
The names of the elements present in the following compounds:
(a) Quick lime (CaO) – Calcium and oxygen
(b) Hydrogen bromide (HBr) – Hydrogen and bromine
(c) Baking powder (NaHCO3)– Sodium, Hydrogen, Carbon, Oxygen
(d) Potassium sulphate (K2SO4) – Potassium, Sulphur, Oxygen
6. Calculate the molar mass of the following substances.
(a) Ethyne, C2H2
(b) Sulphur molecule, S8
(c) Phosphorus molecule, P4 (Atomic mass of phosphorus = 31)
(d) Hydrochloric acid, HCl
(e) Nitric acid, HNO3
Answer:
The molar masses of the substances can be calculated as follows (the unit is in grams):
(a) Molar mass of Ethyne C2H2= 2 x atomic mass of C + 2 x atomic mass of H = (2 × 12) + (2 × 1) = 24 + 2 = 26g.
(b) Molar mass of Sulphur molecule S8 = 8 x Atomic mass of S = 8 x 32 = 256g
(c) Molar mass of Phosphorus molecule, P4 = 4 x Atomic Mass of P = 4 x 31 = 124g
(d) Molar mass of Hydrochloric acid, HCl = Atomic Mass of H + Atomic Mass of Cl = 1 + 35.5 = 36.5g
(e) Molar mass of Nitric acid, HNO3 = Atomic Mass of H + Atomic Mass of N + 3 x Atomic mass of O = 1 + 14 + 3 × 16 = 63g
Solutions to Group Activity (Page No 36) of NCERT Class 9 Science Chapter 3 Atoms and Molecules
Play a game for writing formulae.
Example 1 : Make placards with symbols and valencies of the elements separately. Each student should hold two placards, one with the symbol in the right hand and the other with the valency in the left hand. Keeping the symbols in place, students should criss-cross their valencies to form the formula of a compound.
Example 2 : A low cost model for writing formulae: Take empty blister packs of medicines. Cut them in groups, according to the valency of the element, as shown in the figure. Now, you can make formulae by fixing one type of ion into other.
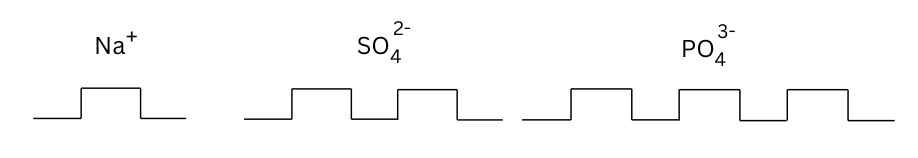
Answer:
Solutions to All Activities of NCERT Class 9 Science Chapter 3 Atoms and Molecules
1. Complete Activity 3.1 (Page 26).
• Take one of the following sets, X and Y of chemicals—
X Y
(i) copper sulphate sodium carbonate
(ii) barium chloride sodium sulphate
(iii) lead nitrate sodium chloride
• Prepare separately a 5% solution of any one pair of substances listed under X and Y each in 10 mL in water.
• Take a little amount of solution of Y in a conical flask and some solution of X in an ignition tube.
• Hang the ignition tube in the flask carefully; see that the solutions do not get mixed. Put a cork on the flask (see Fig. 3.1).
• Weigh the flask with its contents carefully.
• Now tilt and swirl the flask, so that the solutions X and Y get mixed.
• Weigh again.
• What happens in the reaction flask?
• Do you think that a chemical reaction has taken place?
• Why should we put a cork on the mouth of the flask? • Does the mass of the flask and its contents change?
Answer:
2. Complete Activity 3.2 (Page 31).
• Refer to Table 3.4 for ratio by mass of atoms present in molecules and Table 3.2 for atomic masses of elements. Find the ratio by number of the atoms of elements in the molecules of compounds given in Table 3.4.
Answer:
Extra Questions to Complement Solutions to NCERT Class 9 Science Chapter 3 Atoms and Molecules
Very Short Answer Type Questions:
1. You are given a brick. What is the smallest particle of the brick?
Answer:
Atom.
2. Give the radius of the atom of hydrogen in metres.
Answer:
10-10 m.
3. In a chemical reaction, it was found that the mass of reactants = mass of products. Which law of chemical combination governs this phenomenon?
Answer:
Law of conservation of mass.
4. How many atoms of oxygen are present in sulphate ion?
Answer:
Four.
5. What is the atomicity of ozone?
Answer:
3 (Ozone contains 3 atoms of oxygen)
6. Give the symbol of lead.
Answer:
Pb.
7. Give the formula of a diatomic molecule which is essential for respiration.
Answer:
O2.
8. Name two elements which show more than one valency.
Answer:
Iron and copper.
9. Name the British chemist that provided the basic theory about the nature of matter.
Answer:
John Dalton.
10. Give the full form of IUPAC.
Answer:
International Union of Pure and Applied Chemistry.
Multiple Choice Questions (MCQ):
1. What is the anion in sodium chloride?
(A) Cl–
(B) Na+
(C) NaCl
(D) Na–
Answer: (A) Cl–
Anion is the negatively charged anion. In case of sodium chloride, it is Cl–.
2. The valency of aluminium ion is:
(A) + 2
(B) + 3
(C) + 1
(D) – 3
Answer: (B) + 3
3. The total number of atoms in ammonium sulphate is:
(A) 2
(B) 15
(C) 14
(D) 13
Answer: (B) 15
The formula for ammonium sulphate is (NH4)2SO4.
In each molecule of ammonium sulphate, there are:
2 nitrogen atoms (2N)
8 hydrogen atoms (4H x 2)
1 sulphur atom (1S)
4 oxygen atoms (4O)
Adding these up: Total number of atoms in ammonium sulphate = 2N + 8H + 1S + 4O = 2 + 8 + 1 + 4 = 15.
4. An element X has valency = 2. What will its formula be with sulphate ion?
(A) X2SO4
(B) X(SO4)2
(C) XSO4
(D) X2(SO4)2
Answer: (C) XSO4
Valency of element X = 2. Valency of sulphate ion = 2.
Therefore, formula is XSO4.
5. Compound A reacted with compound B in an open conical flask. The mass before and after the reaction was different. Which of the following is correct?
(A) Law of conservation of mass does not hold in this case
(B) The mixture was not stirred properly
(C) Gas was evolved in the reaction
(D) The reaction did not get completed
Answer: (C) Gas was evolved in the reaction
Gas was evolved in the reaction which escaped through the open mouth of the conical flask. As a result, the mass of the products was less than that of the reactants. Law of conservation of mass holds for all reactions.
6. Which of the following symbols of elements is incorrect?
(A) Na
(B) AL
(C) Co
(D) S
Answer: (B) AL
The correct symbol of aluminium is Al.
Short Answer Type Questions:
1. 10g carbonic acid decomposes to form 4g water and carbon dioxide. What is the amount of carbon dioxide formed?
Answer:
By the law of conservations of mass, mass of reactants = mass of products.
Therefore,
Mass of carbonic acid = mass of water + mass of carbon dioxide
or, mass of carbon dioxide = 10g – 4g = 6g.
2. What is the ratio by mass of the two ions in sodium chloride?
Answer:
Atomic mass of sodium = 23 u.
Atomic mass of chlorine = 35.5 u.
Therefore, ratio of mass of the two ions = 23:35.5.
3. It was observed experimentally that 3g of carbon combines with 4g of oxygen to form CO. How much oxygen reacts with all of 12g of carbon to form CO?
Answer:
3g of carbon combines with 4g of oxygen to form CO.
We are required to find how much oxygen combines with all of 12 g of carbon to form CO.
The law of constant proportions states that, “In a compound elements are always present in definite proportions by mass.”
Therefore,
3/4 = 12/(Mass of oxygen)
or, Mass of oxygen = 16g.
Therefore, the mass of oxygen that reacts with all of 12g of carbon to form CO is 16g.
4. Give the valencies of the ions in Calcium oxide.
Answer:
Calcium oxide contains the ions Ca2+ which has valency 2 and O2- which has valency 2.
5. How many atoms are present in
(i) Carbonate ion
(ii) Phosphate ion?
Answer:
(i) Carbonate ion
Symbol is CO32-. Therefore, the number of atoms = 1 C atom + 3 oxygen atoms = 4.
(ii) Sulphate ion?
Symbol is SO42-. Therefore, the number of atoms = 1 sulphur atom + 4 oxygen atoms = 5.
6. Find the formula unit mass of
(i) Sodium sulphate
(ii) Magnesium chloride
Answer:
(i) Sodium sulphate
Na2SO4 = 2 × atomic mass of Na + Atomic mass of sulphur + 4 × atomic mass of oxygen
= 2 × 23 + 32 + 4 × 16 = 142 u.
(ii) Magnesium chloride
MgCl2 = Atomic mass of magnesium + 2 × atomic mass of chlorine
= 24 + 2 × 35.5 = 95 u.
7. Why did scientists initially take 1/16 of the mass of an atom of naturally occurring oxygen as the unit?
Answer:
Scientists initially took 1/16 of the mass of an atom of naturally occurring oxygen as the unit because:
- Oxygen reacted with a large number of elements and formed compounds.
- This atomic mass unit gave masses of the elements as whole numbers.
8. Define one atomic mass unit.
Answer:
One atomic mass unit is a mass unit equal to exactly one-twelfth (1/12th) the mass of one atom of carbon-12.
9. Classify the following on the basis of their atomicity:
(i) CO
(ii) H2O2
(iii) NO2
Answer:
(i) CO
Number of atoms = 1 C atom + 1 O atom = 2. Hence, it is diatomic.
(ii) H2O2
Number of atoms = 2 H atoms + 2 O atoms = 4. Hence, it is tetraatomic.
(iii) NO2
Number of atoms = 1 N atom + 2 O atoms = 3. Hence, it is triatomic.
10. Give the symbol of the following:
(i) Sulphide
(ii) Nitride
Answer:
(i) S2-
(ii) N3-
Fill in the Blanks:
(a) _________ are not able to exist independently and form _________.
(b) The combining power of an element is called its _________.
(c) 1 nm = ______ m.
(d) The Latin name of iron is _________.
(e) Positively charged ions are known as ________ and negatively charged ions are known as _________.
Answers:
(a) Atoms are not able to exist independently and form molecules.
(b) The combining power of an element is called its valency.
(c) 1 nm = 10-9 m.
(d) The Latin name of iron is ferrum.
(e) Positively charged ions are known as cations and negatively charged ions are known as anions.
++++++++++++++
Frequently Asked Questions (FAQs) on NCERT Solutions to Class 9 Science Chapter 3 Atoms and Molecules
Our expert team of Indian and foreign-educated engineers and scientists have answered all in-text questions, exercise questions, group activities and in-text activities in this material, so you can study with complete peace of mind. An extra question set has also been included which resembles exam questions and will give you extra practice.
The free PDFs of the solutions are also available for download anytime! Like what we offer? Want more helpful materials from us? Keep visiting our website and subscribe to our email list to be among the first to access all the latest goodies. (insert hyperlink)
The following topics are covered:
3.1 Laws of Chemical Combination
3.2 What is an Atom?
3.3 What is a Molecule?
3.4 Writing Chemical Formulae
3.5 Molecular Mass
3. How many problems are there in the exercises for NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules?
Here are the number of problems for the chapter:
(i) 2 Numerical Problems (Questions 1, 2)
(ii) 4 Theoretical Questions (Questions 3 – 6)
All the topics in this chapter are equally important and you can expect both numerical and theoretical questions from them in your exams. Practising numericals from this chapter is essential and our extra material is an excellent place to start practising. Our extra material also contains theoretical questions which will clear your concepts and make you confident.
If you need extra coaching, our expert teachers will be here for you anytime you need them. Feel free to contact us anytime and let us help you out! (Insert hyperlink)
In addition to exam preparation, our personable ‘teacher-mentors’ counsel students about their academic and future professional careers. If you have any question or concerns, ask them and they will be happy to guide you. We have found this ‘teacher-mentor’ approach to be very beneficial to students. So, need a friendly teacher-mentor? Reach out to us anytime! We provide expert one-on-one coaching and mentoring to you depending on your convenience and needs – have it completely your way!


