Hello students and welcome to the fundamental chapter on atomic structure! We have answered all in-text questions, exercise questions and in-text activities in this solutions material, to give you a complete learning experience. Our logical and easy-to-follow explanations will help you grasp key ideas with confidence. Plus, take advantage of our bonus set of practice questions to reinforce your learning. With our resources, you will gain the practice you need to excel in your exams.
Solutions to In Text Questions of NCERT Class 9 Science Chapter 4 Structure of the Atom
Page 39:
1. What are canal rays?
Answer:
- Canal rays are positively charged radiations.
- They were identified by E. Goldstein in 1886 as new radiations in a gas discharge.
- These led to the discovery of the sub-atomic particle called the proton.
- They are also called anode rays.
2. If an atom contains one electron and one proton, will it carry any charge or not?
Answer:
If an atom contains one electron and one proton, it will not carry any charge. An electron is a negatively charged particle with charge -1 whereas a proton is a positively charged particle with charge +1. Since the magnitude of the charges are equal and opposite, the atom will not carry any net charge and will be neutral.
Page 41:
1. On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Answer:
On the basis of Thomson’s model of an atom, an atom consists of a positively charged sphere and the electrons are embedded in it. The negative and positive charges are equal in magnitude. So, the positive and negative charges counterbalance each other and the atom as a whole is electrically neutral.
2. On the basis of Rutherford’s model of an atom, which sub atomic particle is present in the nucleus of an atom?
Answer:
On the basis of Rutherford’s model of an atom, the positively charged protons are present in the nucleus of an atom.
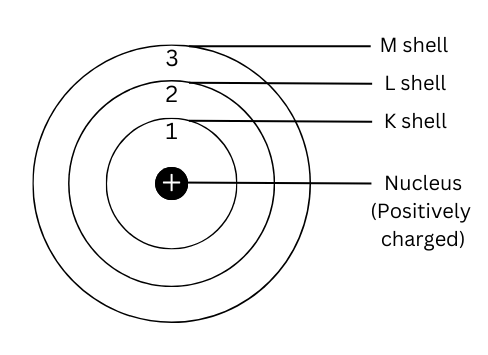
3. Draw a sketch of Bohr’s model of an atom with three shells.
Answer:
A sketch of Bohr’s model of an atom with three shells is shown below:
4. What do you think would be the observation if the α-particle scattering experiment is carried out using a foil of a metal other than gold?
Answer:
If the α-particle scattering experiment is carried out using a foil of a metal other than gold, the observations would remain the same. This is because the basic structure of the atom is the same, irrespective of the material.
Page 41:
1. Name the three sub-atomic particles of an atom.
Answer:
The three sub-atomic particles of an atom are electrons (negatively charged), protons (positively charged) and neutrons (neutrons, no charge).
2. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Answer:
Atomic mass = number of protons + number of neutrons
or, number of neutrons = atomic mass – number of protons
or, number of neutrons = 4u – 2 = 2
Page 42:
1. Write the distribution of electrons in carbon and sodium atoms.
Answer:
From Table 4.1:
The total number of electrons in carbon atom = 6.
The maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is the orbit number or energy level index, 1,2,3,….
Hence the maximum number of electrons in the first orbit or K-shell will be = 2 × 12 = 2.
Therefore, the remaining electrons will be in the second orbit or L-shell = 6 – 2 = 4.
The distribution of electrons in carbon atom can be written as 2, 4.
The total number of electrons in sodium atom = 11.
The maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is the orbit number or energy level index, 1,2,3,….
Hence the maximum number of electrons in the first orbit or K-shell will be = 2 × 12 = 2.
The maximum number of electrons in the second orbit or L-shell will be = 2 × 22 = 8.
Therefore, the remaining electrons will be in the third orbit or M-shell = (11 – 2 – 8) = 1.
The distribution of electrons in carbon atom can be written as 2, 8, 1.
2. If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Answer:
K shell can hold maximum of 2 electrons.
L shell can hold maximum of 8 electrons.
If K and L shells of an atom are full,the total number of electrons present in the atom = 2 + 8 = 10 electrons.
Page 44:
1. How will you find the valency of chlorine, sulphur and magnesium?
Answer:
Chlorine:
The number of electrons in the outermost shell of chlorine = 7.
Therefore, chlorine needs to gain 1 electron to complete a stable octet.
Therefore, valency of chlorine = 8 – 7 = 1.
Sulphur:
The number of electrons in the outermost shell of sulphur = 6.
Therefore, sulphur needs to gain 2 electrons to complete a stable octet.
Therefore, valency of sulphur = 8 – 6 = 2.
Magnesium:
The number of electrons in the outermost shell of magnesium = 2.
Therefore, magnesium needs to lose 2 electrons to complete a stable octet.
Therefore, valency of magnesium = 2.
Page 44:
1. If number of electrons in an atom is 8 and number of protons is also 8, then (i) what is the atomic number of the atom? and (ii) what is the charge on the atom?
Answer:
(i) Atomic number = number of protons. Hence, the atomic number of the atom = 8.
(ii) The number of electrons and protons in the atom are equal (= 8). Electrons and protons have charges of the same magnitude, but opposite in sign. Therefore, the charges counterbalance each other and the net charge on the atom = 0.
2. With the help of Table 4.1, find out the mass number of oxygen and sulphur atom.
Answer:
The mass number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom.
(a) Oxygen
From Table 4.1,
Number of protons of oxygen = 8.
Number of neutrons of oxygen = 8.
Therefore, mass number of oxygen = number of protons + number of neutrons = 8 + 8 = 16.
(b) Sulphur
From Table 4.1,
Number of protons of sulphur = 16.
Number of neutrons of sulphur = 16.
Therefore, mass number of sulphur = number of protons + number of neutrons = 16 + 16 = 32.
Page 45:
1. For the symbol H, D and T tabulate three sub-atomic particles found in each of them.
Answer:
| Isotope | Symbol | Protons | Electrons | Neutrons |
| Hydrogen | H | 1 | 1 | 0 |
| Deuterium | D | 1 | 1 | 1 |
| Tritium | T | 1 | 1 | 2 |
2. Write the electronic configuration of any one pair of isotopes and isobars.
Answer:
One pair of isotopes are![]() .
.
The atomic number of both = 6. Therefore, the electronic configuration of both = 2, 4.
One pair of isobars is ![]() . The atomic number of Ar = 18. Therefore, electronic configuration = 2, 8, 8. The atomic number of Ca = 20. Therefore, electronic configuration = 2, 8, 8, 2.
. The atomic number of Ar = 18. Therefore, electronic configuration = 2, 8, 8. The atomic number of Ca = 20. Therefore, electronic configuration = 2, 8, 8, 2.
Solutions to Exercises (Page No 46) of NCERT Class 9 Science Chapter 4 Structure of the Atom
1. Compare the properties of electrons, protons and neutrons.
Answer:
| Electrons | Protons | Neutrons |
| Negatively charged | Positively charged | Neutral, no charge. |
| Negligible mass | Mass = 1 a.m.u. | Mass = 1 a.m.u. |
| Present in orbits around the nucleus. | Present in the nucleus. | Present in the nucleus. |
2. What are the limitations of J.J. Thomson’s model of the atom?
Answer:
The limitations of J.J. Thomson’s model of the atom are:
- It failed to explain the results of the alpha particle scattering experiment conducted by Rutherford. It failed to explain why most of the alpha particles passed straight through the gold foil, some were deflected by small angles and very few of the particles appeared to rebound.
3. What are the limitations of Rutherford’s model of the atom?
Answer:
The limitations of Rutherford’s model of the atom is that it failed to explain the stability of atoms. The charged electron in a circular orbit would undergo acceleration. During acceleration, charged particles would radiate energy. Thus, the revolving electron would lose energy and finally fall into the nucleus, making the atom highly unstable. Hence matter would not exist in the form that we know.
4. Describe Bohr’s model of the atom.
Answer:
The Bohr’s model of the atom is as follows:
- Atoms consist of a central nucleus containing protons and neutrons.
- Electrons move in circular orbits around the positively charged nucleus.
- Only certain special orbits known as discrete orbits of electrons, are allowed inside the atom.
While revolving in discrete orbits the electrons do not radiate energy.
5. Compare all the proposed models of an atom given in this chapter.
Answer:
| Thomson Model | Rutherford Model | Bohr Model |
| Proposed by J.J. Thomson in 1904. | Proposed by Ernest Rutherford in 1911. | Proposed by Niels Bohr in 1913. |
| Atom consists of a sphere of positive charge with electrons embedded in them. | Electrons revolve around the positively charged nucleus in circular paths called orbits. | Electrons revolve around the positively charged nucleus only in special orbits known as discrete orbits. |
| The whole atom has mass. | Only a small portion called the nucleus at the centre has mass. | Only a small portion called the nucleus at the centre has mass. |
| This model could not explain the results of the Rutherford scattering experiment. | This model was created to explain the results of the Rutherford scattering experiment. | This model was in line with the results of the Rutherford scattering experiment. |
| Electrons are stationary, so could not explain the drawbacks of the Rutherford model. | The drawback of this model was that electrons revolving around circular orbits would radiate energy and fall onto the nucleus, making the atom unstable. | Electrons revolve in special orbits known as discrete orbits and do not radiate energy. Hence, it addressed the drawbacks of the Rutherford model. |
6. Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Answer:
The rules for writing of distribution of electrons in various shells are as follows:
(i) The maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is the orbit number or energy level index, 1,2,3,..
(ii) The maximum number of electrons that can be accommodated in the outermost orbit is 8.
(iii) Electrons are not accommodated in a given shell, unless the inner shells are filled. That is, the shells are filled in a step-wise manner.
7. Define valency by taking examples of silicon and oxygen.
Answer:
The electronic configuration of silicon is 2, 8, 4. Therefore, silicon will gain 4 electrons to complete its octet. Therefore, valency = 8 – 4 = 4.
The electronic configuration of oxygen is 2, 6. It is easier for oxygen to gain two electrons than lose two electrons to complete its octet. Therefore, the valency of oxygen = 8 – 6 = 2.
8. Explain with examples (i) Atomic number, (ii) Mass number, (iii) Isotopes and (iv) Isobars. Give any two uses of isotopes.
Answer:
(i) Atomic number is defined as the number of protons in the nucleus of an atom. For example, for carbon, 6 protons are present in the nucleus, hence the atomic number of carbon = 6.
(ii) The mass number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom. For example, there are 6 protons and 6 neutrons present in the nucleus of carbon. Hence, its mass number is 12.
(iii) Isotopes are atoms of an element which have the same atomic number but different mass numbers. For example, ![]() have same atomic number (= 6) but different mass numbers (12 and 14) and are hence, isotopes.
have same atomic number (= 6) but different mass numbers (12 and 14) and are hence, isotopes.
(iv) Isobars are atoms of elements with different atomic numbers and same mass number. One pair of isobars is ![]() . The atomic numbers are different (18 and 20) but mass number is the same (=40).
. The atomic numbers are different (18 and 20) but mass number is the same (=40).
Uses of Isotopes:
Any two uses of isotopes are:
(i) An isotope of uranium is used as a fuel in nuclear reactors.
(ii) An isotope of cobalt is used in the treatment of cancer.
9. Na+ has completely filled K and L shells. Explain.
Answer:
Na+ has completely filled K and L shells. This is because the number of electrons of sodium = 11 and hence the number of electrons in Na+ is 10. As a result, 2 electrons are present in the K shell and 8 electrons are present in the L shell, thereby completely filling both K and L shells.
10. If bromine atom is available in the form of, say, two isotopes ![]() calculate the average atomic mass of bromine atom.
calculate the average atomic mass of bromine atom.
Answer:![]()
Therefore, average atomic mass of bromine atom = 79 × 49.7/100 + 81 × 50.3/100 = 80.006 u.
11. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes ![]() in the sample?
in the sample?
Answer:
Let the percentages of isotopes ![]() are A% and (100 – A)% respectively.
are A% and (100 – A)% respectively.
The average atomic mass of a sample of an element X is 16.2 u.
Therefore,
16 × A/100 + 18 × (100-A)/100 = 16.2
or, 16A + 18(100 – A) = 1620
or, 1800 – 1620 = 18A – 16A
or, 2A = 180
or, A = 90
100 – A = 10
Therefore, the percentages of isotopes ![]() in the sample are 90% and 10% respectively.
in the sample are 90% and 10% respectively.
12. If Z = 3, what would be the valency of the element? Also, name the element.
Answer:
Atomic number of the element (Z) = 3. The electronic configuration is 2, 1. Since there is only one electron in the outermost shell of the element, it can lose that electron to complete its octet. Therefore, valency = 1. The name of the element is Lithium.
13. Composition of the nuclei of two atomic species X and Y are given as under
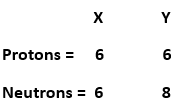
Give the mass numbers of X and Y. What is the relation between the two species?
Answer:
Mass number = number of protons + number of electrons
Therefore,
Mass number of X = 6 + 6 = 12.
Mass number of Y = 6 + 8 = 14.
X and Y have the same atomic number = 6 and different mass numbers. The relation between the two species is that they are isotopes of each other.
14. For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer:
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
False. In J.J Thomson’s model electrons are embedded in a sphere of positive charge. It did not talk about neutrons, nucleons or even the nucleus of the atom.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
False. A neutron is an independent sub-atomic particle and is not formed by an electron and a proton combining together.
(c) The mass of an electron is about 1/2000 times that of proton.
True.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
True. An isotope of iodine, typically iodine-131 (I-131), is used in the production of tincture of iodine, a common antiseptic solution used in medicine.
Put tick (✓) against correct choice and cross (×) against wrong choice in questions 15, 16 and 17
15. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic Nucleus (b) Electron
(c) Proton (d) Neutron
Answer: (a) Atomic Nucleus
Rutherford’s alpha-particle scattering experiment was responsible for the discovery of the nucleus of the atom. He found that most of the space inside an atom is empty and the entire mass is concentrated in a very small area called the nucleus.
16. Isotopes of an element have
(a) the same physical properties
(b) different chemical properties
(c) different number of neutrons
(d) different atomic numbers.
Answer:
(c) different number of neutrons
Isotopes have same atomic number and different mass number.
Mass number = number of protons + number of neutrons
Since number of protons are the same, the number of neutrons must be different for the two isotopes.
17. Number of valence electrons in Cl– ion are:
(a) 16 (b) 8 (c) 17 (d) 18
Answer: (b) 8
Total number of electrons in chlorine is 17. The total number of electrons in Cl– is 18. The electronic configuration of chorine is 2, 8, 8. The number of electrons in the last shell = 8.
18. Which one of the following is a correct electronic configuration of sodium?
(a) 2,8 (b) 8,2,1 (c) 2,1,8 (d) 2,8,1
Answer: (d) 2,8,1
Number of electrons in sodium atom = 11.
Number of electrons in the first orbit or K-shell will be = 2 × 12 = 2.
Number of electrons in the second orbit or L-shell will be = 2 × 22 = 8.
Therefore, the remaining electrons will be in the third orbit or M-shell = (11 – 2 – 8) = 1.
The distribution of electrons in carbon atom can be written as 2, 8, 1.
19. Complete the following table.
| Atomic Number | Mass Number | Number of Neutrons | Number of Protons | Number of Electrons | Name of the Atomic Species |
| 9 | – | 10 | – | – | – |
| 16 | 32 | – | – | – | Sulphur |
| – | 24 | – | 12 | – | – |
| – | 2 | – | 1 | – | – |
| – | 1 | 0 | 1 | 0 | – |
Answer:
| Atomic Number | Mass Number | Number of Neutrons | Number of Protons | Number of Electrons | Name of the Atomic Species |
| 9 | 19 | 10 | 9 | 9 | Fluorine |
| 16 | 32 | 16 | 16 | 16 | Sulphur |
| 12 | 24 | 12 | 12 | 12 | Magnesium |
| 1 | 2 | 1 | 1 | 1 | Deuterium |
| 1 | 1 | 0 | 1 | 0 | Hydrogen ion or proton |
We base our calculations on the following formulae:
Atomic number = Number of Protons = Number of electrons
Mass number = Number of Protons + Number of neutrons
Solutions to All Activities of NCERT Class 9 Science Chapter 4 Structure of the Atom
1. Complete Activity 4.1 (Page 38).
A. Comb dry hair. Does the comb then attract small pieces of paper?
B. Rub a glass rod with a silk cloth and bring the rod near an inflated balloon. Observe what happens.
Answer:
2. Complete Activity 4.2 (Page 42).
• Make a static atomic model displaying electronic configuration of the first eighteen elements.
• The composition of atoms of the first eighteen elements is given in Table 4.1.
Answer:
Extra Questions to Complement Solutions to NCERT Class 9 Science Chapter 4 Structure of the Atom
Very Short Answer Type:
1. Who discovered the electron?
Answer:
J.J. Thomson.
2. Who discovered canal rays?
Answer:
E. Goldstein.
3. What is the mass of a proton in a.m.u.?
Answer:
1 amu.
4. Which model of the atom solved the drawbacks of the Rutherford’s atomic model?
Answer:
Bohr’s model of atom.
5. What is the mass of alpha-particles?
Answer:
4 u.
6. Who is known as the ‘Father’ of nuclear physics?
Answer:
Ernest Rutherford.
7. Name the sub-atomic particle with mass equal to that of a proton.
Answer:
Neutron.
8. Name the element which has only one proton.
Answer:
Hydrogen.
9. Name the isotope of hydrogen which has mass number 3.
Answer:
Tritium.
10. In an atom, number of electrons is 7 and number of protons is 8. What is the net charge on the atom?
Answer:
+1
Multiple Choice Questions (MCQ):
1. What is the maximum number of electrons that can be accommodated in the M-shell of an atom?
(A) 2
(B) 8
(C) 10
(D) 18
Answer: (D) 18
The maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is the orbit number or energy level index, 1,2,3,….
Therefore, maximum number of electrons in third orbit or M-shell will be = 2 × 32 = 18.
2. What is the electronic configuration of Al3+?
(A) 2, 8
(B) 2, 8, 3
(C) 2, 8, 2
(D) 2, 8, 1
Answer: (A) 2, 8
The number of electrons in Al3+ is 13.
The electronic configuration of Al is 2, 8, 3.
Al3+ has lost 3 electrons from its valence shell.
Hence, its configuration is 2, 8.
3. What is the valency of Magnesium?
(A) 5
(B) 4
(C) 2
(D) 1
Answer: (C) 2
The number of electrons in Magnesium is 12.
The electronic configuration is 2, 8, 2.
Magnesium has the tendency to lose 2 electrons to form a stable octet. Hence, its combining capacity or valency = 2.
4. What is true about helium, neon and argon?
(A) All have valency 0
(B) All have 8 electrons in their outermost shell
(C) They combine with many elements to form compounds
(D) All have 3 shells in which electrons are distributed
Answer: (A) All have valency 0
5. An element with only 2 electrons would be
(A) Having a stable octet
(B) Inert in nature
(C) Be very reactive
(D) Have valency 2
Answer: (B) Inert in nature
6. Which model of the atom first predicted that an atom is electrically neutral?
(A) Thomson’s Model
(B) Rutherford’s Model
(C) Bohr’s Model
(D) Dalton’s Model
Answer: (A) Thomson’s Model
Short Answer Type:
1. What property of atoms does the phenomenon of electricity indicate?
Answer:
The phenomenon of electricity is due to charged particles. Therefore, it indicates that atoms are divisible and consists of charged sub-atomic particles.
2. Rutherford selected a gold foil because he wanted as thin a layer as possible. Why?
Answer:
Rutherford wanted a thin layer of atoms so that the alpha particles could penetrate the foil and interact with the atoms within it. Thinner foils allowed for a greater chance of alpha particles passing through and interacting with the atoms.
3. The ion of an element has 1 negative charge. Mass number of the atom is 35 and the number of neutrons is 18. What is the number of electrons in the ion? Can you identify the ion?
Answer:
Mass number = number of protons + number of neutrons
Therefore,
Number of protons = Mass number – number of neutrons
or, Number of protons = 35 – 18 = 17
Number of electrons = number of protons = 17.
Electronic configuration is 2, 8, 7.
The atom will gain 1 negative charge to complete its stable octet.
Therefore, the electronic configuration of the ion is 2, 8, 8.
Therefore, the number of electrons in the ion = 2 + 8 + 8 = 18.
The ion is Cl–.
4. An atom of an element has 7 protons and 7 neutrons. What is the mass of the atom of the element?
Answer:
Mass of an atom resides entirely in its nucleus in the form of protons and neutrons.
An atom of the element has 7 protons and 7 neutrons.
Therefore,
Mass of the atom = 7u + 7u = 14 u.
5. The number of protons in an element X is 15 and number of neutrons is 16. Write the element is scientific notation.
Answer:
Mass number = number of protons + number of neutrons = 15 + 16 = 31. The element can be written in proper scientific notation as ![]()
6. Atoms with one or two valence electrons are highly reactive. Explain.
Answer:
If atoms have only one or two valence electrons, they can easily be removed to attain a stable octet. Therefore, the tendency of the atom will be to lose those electrons by reacting with other atoms to attain stability. Hence, they are highly reactive.
7. Which aspect of Dalton’s atomic theory failed and why?
Answer:
Dalton stated that the atom was indivisible and indestructible. This aspect of Dalton’s atomic theory was disproved by the discovery of the sub-atomic particles, the electron and the proton.
8. Which element has a higher valency: nitrogen or oxygen?
Answer:
Atomic number of nitrogen = 7. Electronic configuration of nitrogen is 2, 5. Therefore, it will tend to gain three electrons to attain a stable octet. This gives us valency = 8 – 5 = 3.
Atomic number of oxygen = 8. Electronic configuration of oxygen is 2, 6. Therefore, it will tend to gain two electrons to attain a stable octet. This gives us valency = 8 – 6 = 2.
Therefore, nitrogen has a higher valency.
9. How is the mass of atom of an element with three isotopes calculated?
Answer:
If an element occurs in isotopic forms, then we have to know the percentage of each isotopic form and then the average mass is calculated.
10. Give an example of a metal and a non-metal with valency 1.
Answer:
A metal with valency 1 is sodium and a non-metal with valency 1 is chorine.
Long Answer Type:
1. An element X occurs in nature in 3 isotopic forms, with masses 20 u, 22 u and 24 u in the ratio 2:2:1. What is the average mass of X?
Answer:
The ratio of the 3 isotopic forms with masses 20 u, 22 u and 24 uis 2:2:1.
Let us find out the percentages of the isotopes.
Percentage of isotope with mass 20 u = 2/((2 + 2 + 1)) × 100 = 40%.
Percentage of the isotope with mass 22 u = 2/((2 + 2 + 1)) × 100 = 40%.
Percentage of the isotope with mass 24 u = 1/((2 + 2 + 1)) × 100 = 20%.
Therefore, the average mass of X is = 20 u × 40/100 + 22u × 40/100 + 24 u × 20/100 = 21.6 u.
Fill in the Blanks:
(a) The _________ properties of isotopes are similar and _________ properties are different.
(b) The valency of neon is _________.
(c) J.J. Thomson directed the _________ laboratory at Cambridge.
(d) Radius of atom is _________ times more than the radius of the nucleus.
(e) Bohr improved Rutherford’s model by saying that electrons revolve around the nucleus in _________ orbits.
Answer:
(a) The chemical properties of isotopes are similar and physical properties are different.
(b) The valency of neon is zero.
(c) J.J Thomson directed the Cavendish laboratory at Cambridge.
(d) Radius of atom is 105 times more than the radius of the nucleus.
(e) Bohr improved Rutherford’s model by saying that electrons revolve around the nucleus in discrete orbits.
Match and Pair:
| Column A | Column B |
| (i) Valency | (a) Highly reactive |
| (ii) Sodium | (b) Isobars |
| (iii) Ca and Ar | (c) Isotopes |
| (iv) | (d) Neutrons |
| (v) J. Chadwick | (e) Combining capacity |
Answer:
| Column A | Column B |
| (i) Valency | (e) Combining capacity |
| (ii) Sodium | (a) Highly reactive |
| (iii) Ca and Ar | (b) Isobars |
| (iv) | (c) Isotopes |
| (v) J. Chadwick | (d) Neutrons |
++++++++++++++
Frequently Asked Questions (FAQs) on NCERT Solutions to Class 9 Science Chapter 4 Structure of the Atom
Our team has thoroughly answered all in-text questions, exercise questions and in-text activities in this material, so you can access the solutions to everything in one place. Attractive figures have been included wherever necessary. We recommend that you study the extra question set as well as it resembles exam questions and will give you extra practice.
The free PDFs of the solutions are also available for download anytime! If you like our material and want more from us, keep visiting our website and subscribe to our email list to stay updated with the latest materials and exclusive offers. (insert hyperlink)
The following topics are covered:
4.1 Charged Particles in Matter
4.2 The Structure of an Atom
4.3 How are Electrons Distributed in Different Orbits (Shells)?
4.4 Valency
4.5 Atomic Number and Mass Number
4.6 Isotopes
Here are the number of problems for the chapter:
(i) 11 Theoretical Questions (Questions 1 – 9, 12, 13)
(ii) 2 Numerical Questions (Questions 10, 11)
(iii) 1 True/False Question (Questions 14)
(iv) 4 Multiple Choice Questions (Questions 15 – 18)
(v) 1 Complete the Table Questions (Question 19)
Atomic structure is a very fundamental chapter which you will use throughout your academic career in science. Therefore, it is essential that you understand and learn all the concepts in this chapter equally well. We recommend you test your understanding and preparation using the extra problem set we have included.
Do you need extra coaching? We will be there for you anytime you need us! Feel free to reach out to us anytime and let us help you out! (Insert hyperlink)
In addition to teaching, we counsel students regarding their future academic and professional careers as a free service. Have any questions or concerns? Ask us, we are happy to help! We have found this ‘teacher-mentor’ approach to be very beneficial to students. If you like what we offer, feel free to reach out to us anytime! We provide expert one-on-one coaching and mentoring to you depending on your convenience and needs – have it completely your way!


