10. Complete Activity 2.10 (Page 24).
- Take 10 mL water in a beaker.
- Add a few drops of concentrated H2SO4 to it and swirl the beaker slowly.
- Touch the base of the beaker.
- Is there a change in temperature?
- Is this an exothermic or endothermic process?
- Repeat the above Activity with sodium hydroxide pellets and record your observations.
Answer:
Aim: To add concentrated H2SO4 and sodium hydroxide pellets to water and conclude based on the observations.
Materials Required: Water, beaker, concentrated H2SO4, sodium hydroxide pellets.
Procedure:
(i) Pour 10 mL of water in the beaker.
(ii) Add a few drops of concentrated H2SO4 tothe beaker while slowly swirling it.
(iii) Touch the base of the beaker and note what happens.
(iv) Repeat the activity by dissolving sodium hydroxide pellets in water and note the observations.
Observations:
- On addition of concentrated H2SO4 tothe beaker, the base of the beaker feels warm.
- On dissolution of sodium hydroxide pellets in water, the base of the beaker feels warm as well.
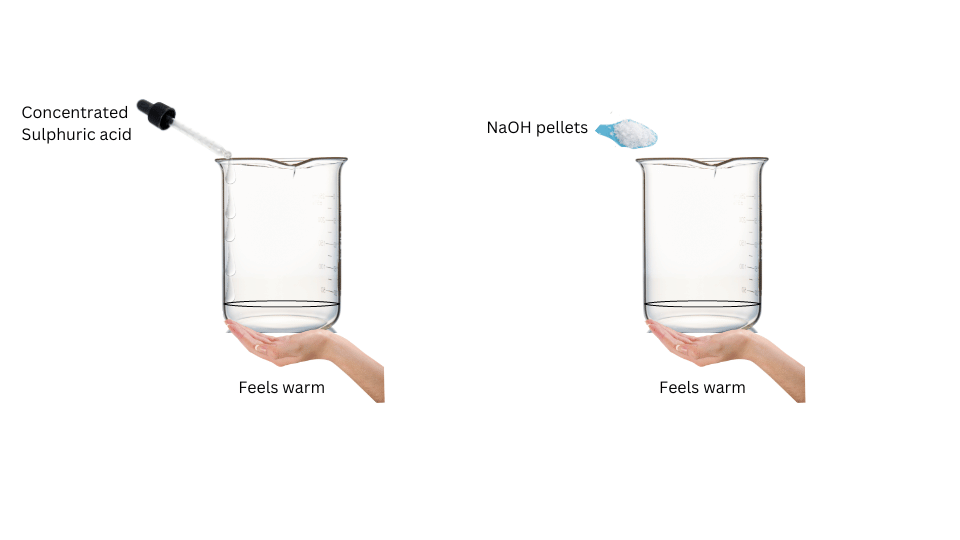
Conclusions:
The process of dissolving an acid or base in water is an exothermic process.
“10. Complete Activity 2.10 (Page 24).
- Take 10 mL water in a beaker.
- Add a few drops of concentrated H2SO4 to it and swirl the beaker slowly.
- Touch the base of the beaker.
- Is there a change in temperature?
- Is this an exothermic or endothermic process?
- Repeat the above Activity with sodium hydroxide pellets and record your observations.“ – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 2.1
Solution to Activity 2.2
Solution to Activity 2.3
Solution to Activity 2.4
Solution to Activity 2.5
Solution to Activity 2.6
Solution to Activity 2.7
Solution to Activity 2.8
Solution to Activity 2.9
Solution to Activity 2.10
Solution to Activity 2.11
Solution to Activity 2.12
Solution to Activity 2.13
Solution to Activity 2.14
Solution to Activity 2.15


