
Answer:
Aim: To observe if the water is still coloured after diluting the solution 5-8 times and conclude based on what you observe.
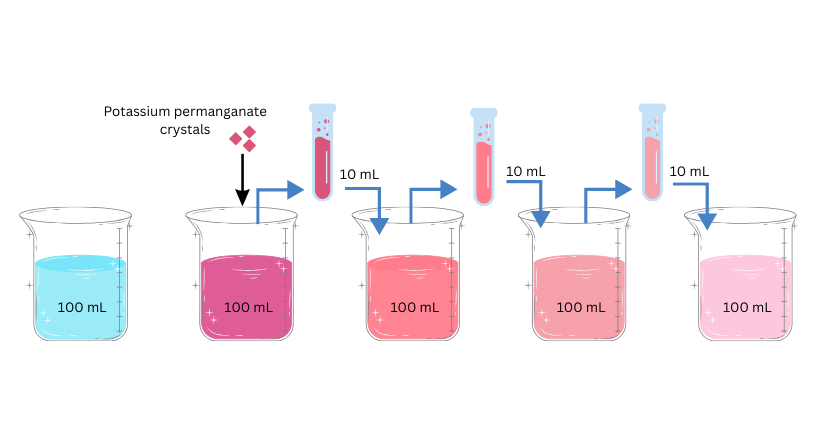
Materials Required: 2–3 crystals of potassium permanganate, 100 mL of water,5 beakers, test tube.
Procedure:
(i) Pour 100 mL of water in a beaker.
(ii) Dissolve the 2-3 crystals of potassium permanganate in the beaker.
(iii) Now extract approximately 10 mL of this solution from the first beaker and put it into 90 mL of clear water in the second beaker.
(iv) Now extract 10 mL of this solution from the second beaker and put it into another 90 mL of clear water in the third beaker.
(v) Keep diluting the solution 5 to 8 times and at the end note if the water is still coloured.
Observations: You will observe that at the end the water is still lightly coloured.
Conclusions:
- This shows that just a few crystals of potassium permanganate is enough to colour a large volume of water (approximately 100 mL).
- There must be millions of tiny particles inside one crystal of potassium permanganate which spread into a large volume of water and colour it.
- Therefore, we conclude matter is made of millions of tiny particles.

Related Links:
Solution to Group Activity
Solution to Activity 1.1
Solution to Activity 1.3
Solution to Activity 1.4
Solution to Activity 1.5
Solution to Activity 1.6
Solution to Activity 1.7
Solution to Activity 1.8
Solution to Activity 1.9
Solution to Activity 1.10
Solution to Activity 1.11
Solution to Activity 1.12
Solution to Activity 1.13
Solution to Activity 1.14
Solutions to Chapter 1 Matter in Our Surroundings


