12. Complete Activity 3.12 (Page 44).
- Take a clean wire of copper and an iron nail.
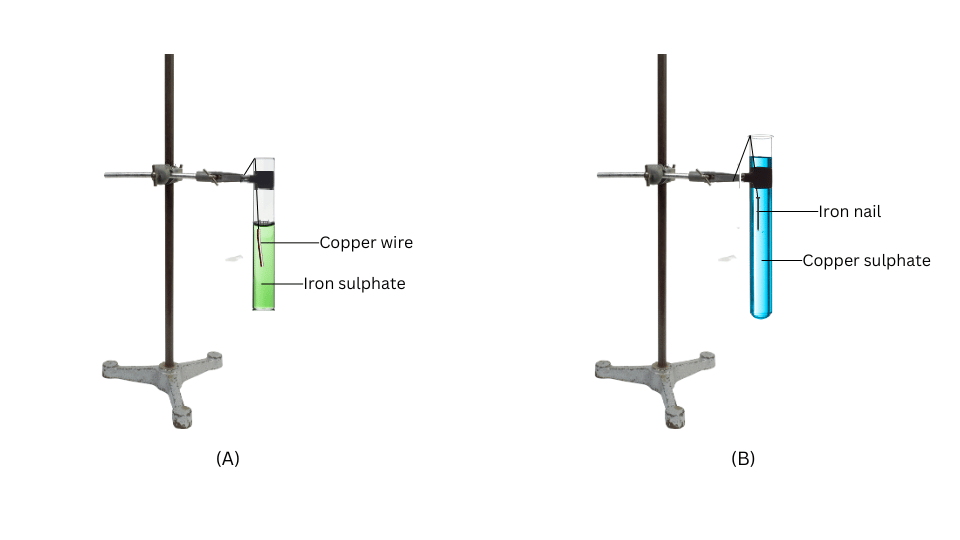
- Put the copper wire in a solution of iron sulphate and the iron nail in a solution of copper sulphate taken in test tubes (Fig. 3.4).
- Record your observations after 20 minutes.
- In which test tube did you find that a reaction has occurred?
- On what basis can you say that a reaction has actually taken place?
- Can you correlate your observations for the Activities 3.9, 3.10 and 3.11?
- Write a balanced chemical equation for the reaction that has taken place.
- Name the type of reaction.
Answer:
Aim: To place the copper wire and iron nail in solutions of iron sulphate and copper sulphate respectively and based on the observations make the necessary conclusions.
Materials Required: Copper wire, iron nail, iron sulphate, copper sulphate, two test tubes, two laboratory stands.
Procedure:
(i) Attach the two test tubes to the two laboratory stands.
(ii) Take iron sulphate solution in the first test tube and submerge the copper wire in it using a string.
(iii) Take copper sulphate solution in the second test tube and submerge the iron nail in it using a string.
(iv) After 20 minutes record your observations.
Observations:
- There is no change in the first test tube.
- In the second test tube, the colour of the solution fades and a brown coating appears on the iron nail.
Conclusions:
- A reaction has occurred in the test tube containing the iron nail and copper sulphate solution as is indicated by the change in colour and brown deposit on the iron nail.
- As was proved in Activities 3.10 and 3.11, iron is more reactive than copper. Hence, iron displaced copper from copper sulphate solution to form iron sulphate solution and the metallic copper got deposited on the iron nail.
- The balanced chemical equation for the reaction is: Fe(s) + CuSO4(aq) —> FeSO4(aq) + Cu(s)
- The reaction is called a displacement reaction.
“12. Complete Activity 3.12 (Page 44).
- Take a clean wire of copper and an iron nail.
- Put the copper wire in a solution of iron sulphate and the iron nail in a solution of copper sulphate taken in test tubes (Fig. 3.4).
- Record your observations after 20 minutes.
- In which test tube did you find that a reaction has occurred?
- On what basis can you say that a reaction has actually taken place?
- Can you correlate your observations for the Activities 3.9, 3.10 and 3.11?
- Write a balanced chemical equation for the reaction that has taken place.
- Name the type of reaction.” – Solved.
Related Links:
Solution to Activity 3.1
Solution to Activity 3.2
Solution to Activity 3.3
Solution to Activity 3.4
Solution to Activity 3.5
Solution to Activity 3.6
Solution to Activity 3.7
Solution to Activity 3.8
Solution to Activity 3.9
Solution to Activity 3.10
Solution to Activity 3.11
Solution to Activity 3.12
Solution to Activity 3.13
Solution to Activity 3.14


