8. Complete Activity 3.8 (Page 40).
- Take a magnesium ribbon and some sulphur powder.
- Burn the magnesium ribbon. Collect the ashes formed and dissolve them in water.
- Test the resultant solution with both red and blue litmus paper.
- Is the product formed on burning magnesium acidic or basic?
- Now burn sulphur powder. Place a test tube over the burning sulphur to collect the fumes produced.
- Add some water to the above test tube and shake.
- Test this solution with blue and red litmus paper.
- Is the product formed on burning sulphur acidic or basic?
- Can you write equations for these reactions
Answer:
Aim: To burn magnesium ribbon and sulphur powder, add water to the resulting products, test the resultant solutions using litmus paper and conclude based on the observations.
Materials Required: Magnesium ribbon, tongs, Bunsen burner, watch glass, beaker, water, red and blue litmus paper, sulphur powder, test tube.
Procedure:
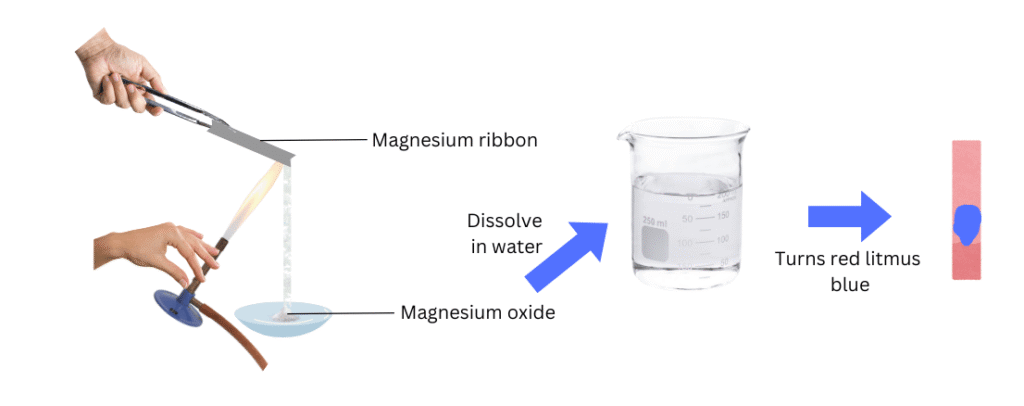
(i) Hold the magnesium ribbon with a pair of tongs and burn it using the Bunsen burner.
(ii) Collect the ashes in a watch glass placed below the burning ribbon and dissolve the ashes in water.
(iii)Test the resulting solution with both red and blue litmus paper and note the observations.
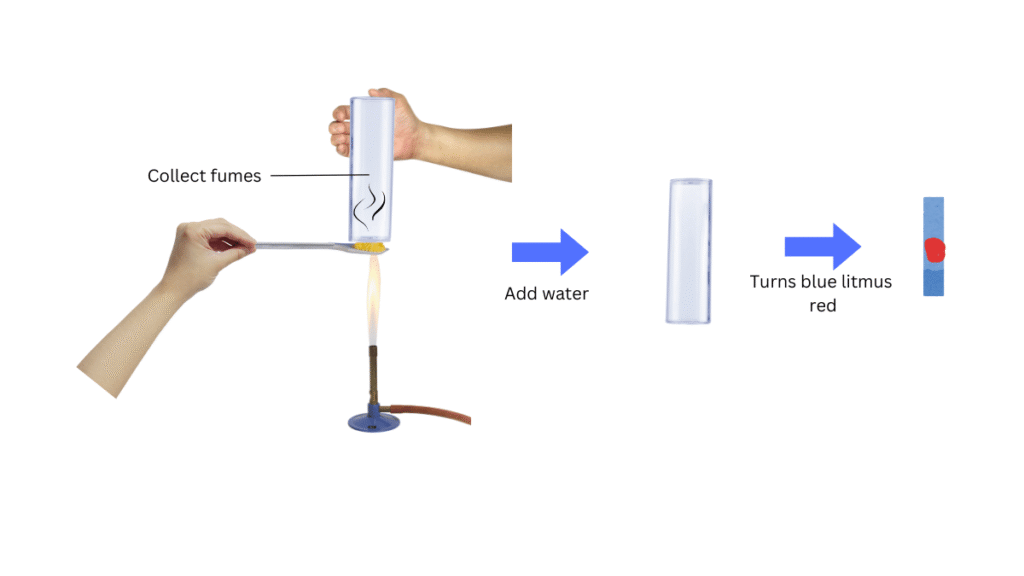
(iv) Now take some sulphur powder in the spatula and hold it right above the burner.
(v) Place a test tube above the burning sulphur to collect the fumes.
(vi) Next, add water to the test tube, shake well and test the resulting solution with both red and blue litmus paper. Note the observations.
(vii) Write equations for the above reactions.
Observations:
When ashes from the magnesium ribbon were dissolved in water, the resulting solution turned red litmus blue.
When water was added to fumes of burning sulphur, the resulting solution turned blue litmus red.
Conclusions:
Metals like magnesium give basic oxides as is indicated by the change of red litmus to blue when the oxide is dissolved in water. The reactions for the experiment are shown below:
2Mg(s) + O2(g) + Heat —> 2MgO(s)
MgO(s) + H2O(l) —> Mg(OH)2(aq)
Non metals like sulphur form acidic oxides as is indicated by the change of blue litmus to red when the oxide is dissolved in water. The reactions for the experiment are shown below:
S(s) + O2(g) + Heat —> SO2(g)
SO2(g) + H2O(l) —> H2SO3(l)
“8. Complete Activity 3.8 (Page 40).
- Take a magnesium ribbon and some sulphur powder.
- Burn the magnesium ribbon. Collect the ashes formed and dissolve them in water.
- Test the resultant solution with both red and blue litmus paper.
- Is the product formed on burning magnesium acidic or basic?
- Now burn sulphur powder. Place a test tube over the burning sulphur to collect the fumes produced.
- Add some water to the above test tube and shake.
- Test this solution with blue and red litmus paper.
- Is the product formed on burning sulphur acidic or basic?
- Can you write equations for these reactions” – Solved.
Related Links:
Solution to Activity 3.1
Solution to Activity 3.2
Solution to Activity 3.3
Solution to Activity 3.4
Solution to Activity 3.5
Solution to Activity 3.6
Solution to Activity 3.7
Solution to Activity 3.8
Solution to Activity 3.9
Solution to Activity 3.10
Solution to Activity 3.11
Solution to Activity 3.12
Solution to Activity 3.13
Solution to Activity 3.14


