7. Complete Activity 2.7 (Page 21).
- Take a small amount of copper oxide in a beaker and add dilute hydrochloric acid slowly while stirring.
- Note the colour of the solution. What has happened to the copper oxide?
Answer:
Aim: To add dilute hydrochloric acidto copper oxide and conclude based on the observations.
Materials Required: Copper oxide, dilute hydrochloric acid, beaker, glass rod, dropper.
Procedure:
(i) Take a small amount of copper oxide in the beaker.
(ii) Using the dropper add dilute hydrochloric acid slowly while stirring. Note the changes in the colour of the solution.
Observations:
The colour of the solution turns blue-green and copper oxide dissolves.
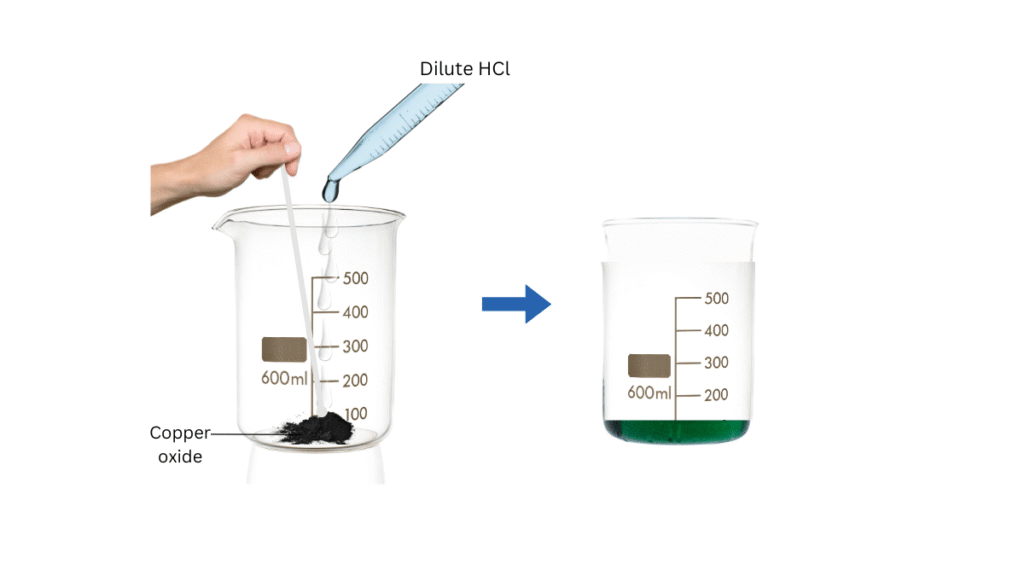
Conclusions:
- The blue-green colour of the solution is due to the formation of copper (II) chloride in the reaction:
CuO + 2HCl —> CuCl2 + H2O - The above result can be generalised to conclude that a metal oxide reacts with an acid to form salt and water: Metal oxide + Acid —> Salt + Water
“7. Complete Activity 2.7 (Page 21).
Note the colour of the solution. What has happened to the copper oxide?
Take a small amount of copper oxide in a beaker and add dilute hydrochloric acid slowly while stirring.” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 2.1
Solution to Activity 2.2
Solution to Activity 2.3
Solution to Activity 2.4
Solution to Activity 2.5
Solution to Activity 2.6
Solution to Activity 2.7
Solution to Activity 2.8
Solution to Activity 2.9
Solution to Activity 2.10
Solution to Activity 2.11
Solution to Activity 2.12
Solution to Activity 2.13
Solution to Activity 2.14
Solution to Activity 2.15


