2. Complete Activity 4.2 (Page 40).
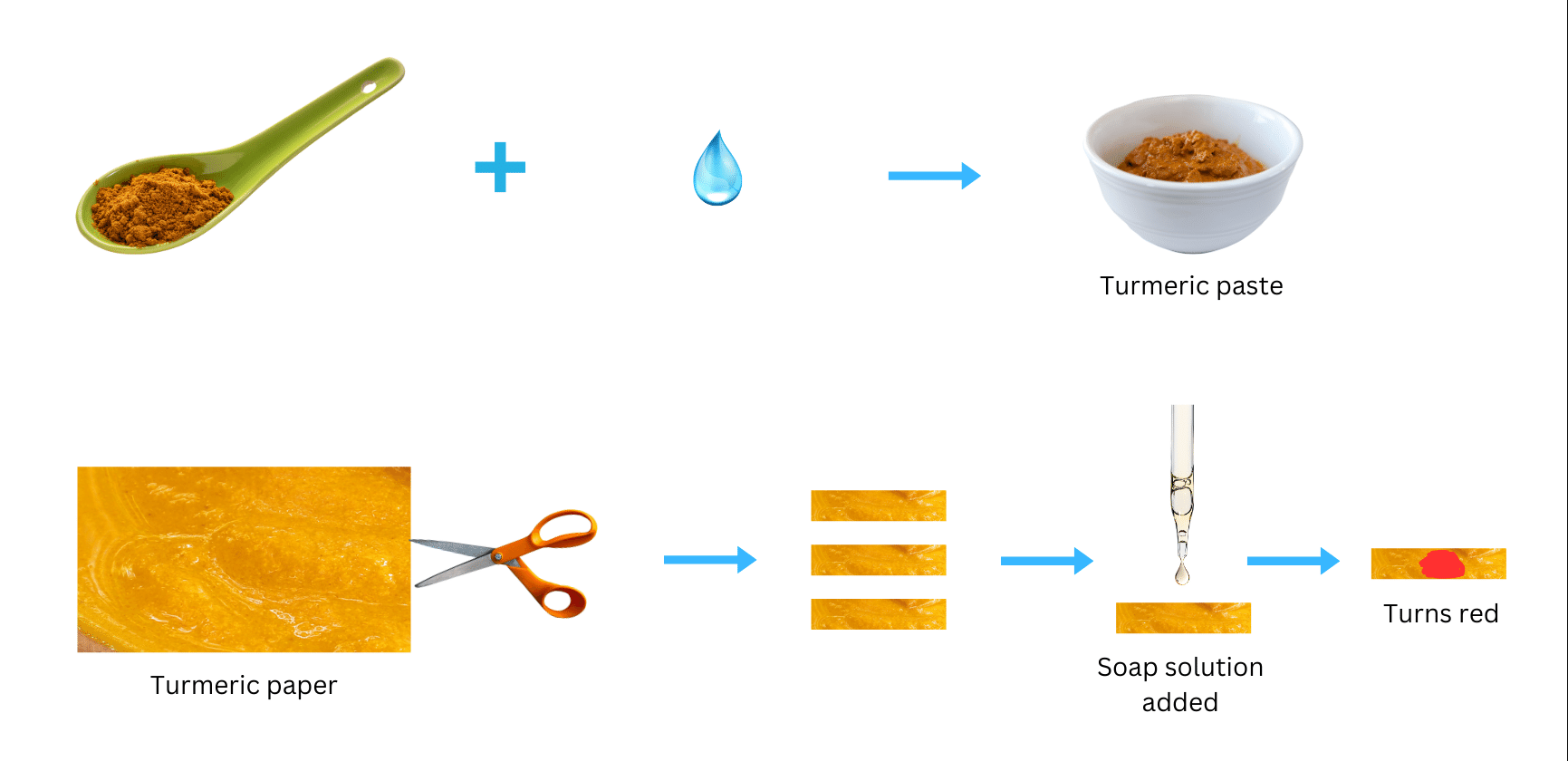
- Take a tablespoonful of turmeric powder. Add a little water and make a paste.
- Make turmeric paper by depositing turmeric paste on blotting paper/filter paper and drying it. Cut thin strips of the yellow paper obtained.
- Put a drop of soap solution on the strip of turmeric paper. What do you observe?
Similarly test the solutions listed in Table 4.3 and note down your observations. You may try solutions of other substances also.
Answer:
The activity is described below:
Aim: To test the solutions in Table 4.3 with turmeric indicator and make conclusions.
Materials Required: Lemon juice, orange juice, vinegar, milk of magnesia, baking soda, lime water, sugar, common salt.
Procedure:
(i) Take a spoon of turmeric powder, add a little bit of water and turn it into a paste.
(ii) Uniformly distribute the turmeric paste on blotting paper/filter and allow it to dry. Cut out at least 9-10 thin strips of yellow turmeric paper from it.
(iii) Put a drop of soap solution on a strip of turmeric paper and observe what happens.
(iv) Similarly test each solution listed in Table 5.3 on a new strip of turmeric paper one by one and observe the results.
Observations:
We observe that soap solution turns the turmeric indicator to red.
The completed Table 4.3 is shown below:
| S. No. | Test Solution | Effect on turmeric solution | Remarks |
| 1. | Lemon juice | Does not change colour | Acidic or neutral |
| 2. | Orange juice | Does not change colour | Acidic or neutral |
| 3. | Vinegar | Does not change colour | Acidic or neutral |
| 4. | Milk of magnesia | Turns red | Basic |
| 5. | Baking soda | Turns red | Basic |
| 6. | Lime water | Turns red | Basic |
| 7. | Sugar | Does not change colour | Acidic or neutral |
| 8. | Common salt | Does not change colour | Acidic or neutral |
Conclusions:
We conclude that:
- Turmeric indicator turns red on contact with basic solutions.
- If there is no change in the colour of turmeric indicator, then the solution may be acidic or neutral.
“Complete Activity 4.2 (Page 40).
- Take a tablespoonful of turmeric powder. Add a little water and make a paste.
- Make turmeric paper by depositing turmeric paste on blotting paper/filter paper and drying it. Cut thin strips of the yellow paper obtained.
- Put a drop of soap solution on the strip of turmeric paper. What do you observe?
Similarly test the solutions listed in Table 4.3 and note down your observations. You may try solutions of other substances also.” – Solved
Related Links:
Solution to Extended Learning Question 1
Solution to Extended Learning Question 2
Solution to Extended Learning Question 3
Solution to Extended Learning Question 4
Solution to Activity 4.1
Solution to Activity 4.3
Solution to Activity 4.4
Solution to Chapter 4 Acids, Bases and Salts


