
Answer:
Aim: To record the observations for conversion of ice to water and then water to vapour and make the necessary conclusions.
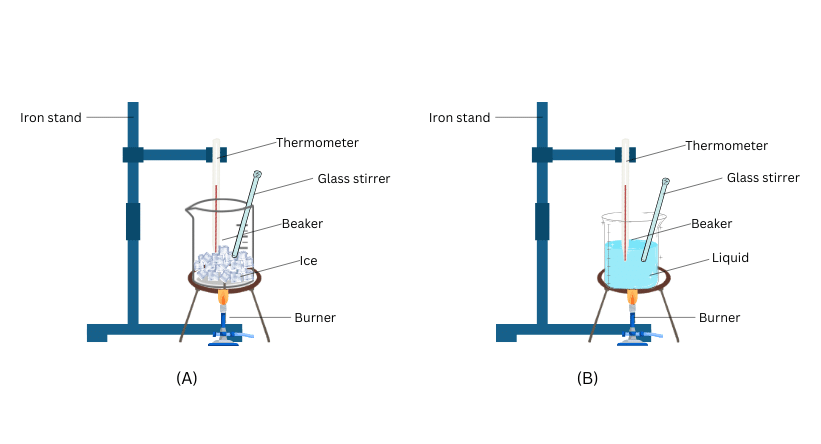
Materials Required: 150 g of ice, beaker, laboratory thermometer, Bunsen burner, glass rod, iron stand.
Procedure:
(i) Put 150 g of ice in the beaker.
(ii) Fix the laboratory thermometer to the iron stand and suspend it in the beaker such that the bulb is in contact with the ice.
(iii) Start heating the beaker on a low flame and note the melting temperature of ice.
(iv) Note the temperature when all the ice has converted into water.
(v) Now heat the water in the beaker and stir with the glass rod until the water starts boiling. (vi) Note the temperature when most of the water has vaporised.
Observations:
- Ice starts melting at 0oC.
- When all the ice has melted into water the temperature is still 0oC.
- When the water is heated in the beaker, its temperature increases until it starts boiling.
- Water starts boiling at 100oC.
- The temperature remains constant at 100oC until all the water has vapourised.
Conclusions:
- Temperature remains constant during change of state.
- During change of state, the heat energy is used up in changing the state by overcoming the forces of attraction between the particles. Hence, there is no rise in temperature.

Related Links:
Solution to Group Activity
Solution to Activity 1.1
Solution to Activity 1.2
Solution to Activity 1.3
Solution to Activity 1.4
Solution to Activity 1.5
Solution to Activity 1.6
Solution to Activity 1.7
Solution to Activity 1.8
Solution to Activity 1.9
Solution to Activity 1.10
Solution to Activity 1.11
Solution to Activity 1.13
Solution to Activity 1.14
Solutions to Chapter 1 Matter in Our Surroundings


