9. Complete Activity 2.9 (Page 23).
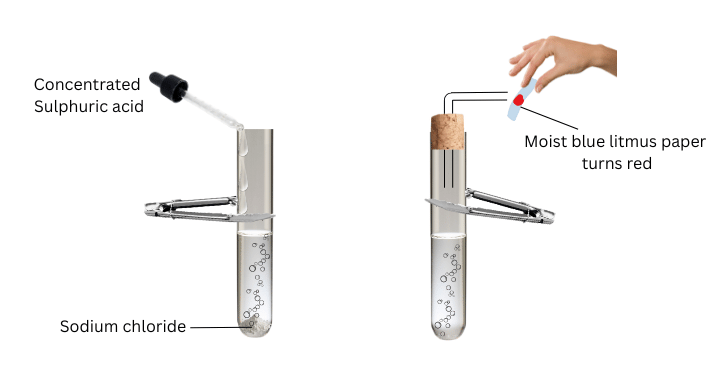
- Take about 1g solid NaCl in a clean and dry test tube and set up the apparatus as shown in Fig. 2.4.
- Add some concentrated sulphuric acid to the test tube.
- What do you observe? Is there a gas coming out of the delivery tube?
- Test the gas evolved successively with dry and wet blue litmus paper.
- In which case does the litmus paper change colour?
- On the basis of the above Activity, what do you infer about the acidic character of: (i) dry HCl gas (ii) HCl solution?
Answer:
Aim: To carry out the reaction between NaCl and concentrated sulphuric acid and conclude based on the observations.
Materials Required: Test tube, tongs, cork, delivery tube, dropper, 1g solid NaCl,concentrated sulphuric acid, dry and wet blue litmus paper.
Procedure:
(i) Place the delivery tube through the cork as shown.
(ii) Now take 1 g solid NaCl in the test tube.
(iii) Add concentrated sulphuric acid to the test tube using a dropper.
(iv) Close the mouth of the test tube with the cork.
(v) Successively hold dry and wet blue litmus paper in front of the delivery tube. Note the observations.
Observations:
- When concentrated sulphuric acid is added to the test tube bubbles are observed.
- A colourless gas is observed to come out of the delivery tube.
- The gas did not change the colour of dry blue litmus paper.
- The gas only changes the colour of wet blue litmus paper to red.
Conclusions:
- The gas evolved in the reaction is HCl.
- Dry HCl does not generate H+ ions and since the blue litmus paper is also dry, the colour does not change.
- Dry HCl gas generates H+ ions on contact with wet blue litmus paper. Hence, the colour of the wet blue litmus paper changes to red.
- We conclude that acids produce H+ ions only in the presence of water.
“9. Complete Activity 2.9 (Page 23).
On the basis of the above Activity, what do you infer about the acidic character of: (i) dry HCl gas (ii) HCl solution?
Take about 1g solid NaCl in a clean and dry test tube and set up the apparatus as shown in Fig. 2.4.
Add some concentrated sulphuric acid to the test tube.
What do you observe? Is there a gas coming out of the delivery tube?
Test the gas evolved successively with dry and wet blue litmus paper.
In which case does the litmus paper change colour?” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 2.1
Solution to Activity 2.2
Solution to Activity 2.3
Solution to Activity 2.4
Solution to Activity 2.5
Solution to Activity 2.6
Solution to Activity 2.7
Solution to Activity 2.8
Solution to Activity 2.9
Solution to Activity 2.10
Solution to Activity 2.11
Solution to Activity 2.12
Solution to Activity 2.13
Solution to Activity 2.14
Solution to Activity 2.15


