9. Carry out the following Activity (Page 10):
Take about 2 g barium hydroxide in a test tube. Add 1 g of ammonium chloride and mix with the help of a glass rod. Touch the bottom of the test tube with your palm. What do you feel? Is this an exothermic or endothermic reaction?
Answer:
Aim: To observe if the reaction between barium hydroxide and ammonium chloride is exothermic or endothermic and conclude based on that.
Materials Required: 2 g barium hydroxide, 1 g of ammonium chloride, glass rod, test tube, spatula.
Procedure:
(i) Using a spatula put 2 g of barium hydroxide in the test tube.
(ii) Next, using the spatula put 1 g of ammonium chloride in the test tube.
(iii) Now, mix with the help of a glass rod.
(iv) Touch the bottom of the test tube with your palm and observe what you feel.
Observations: The bottom of the test tube becomes cold.
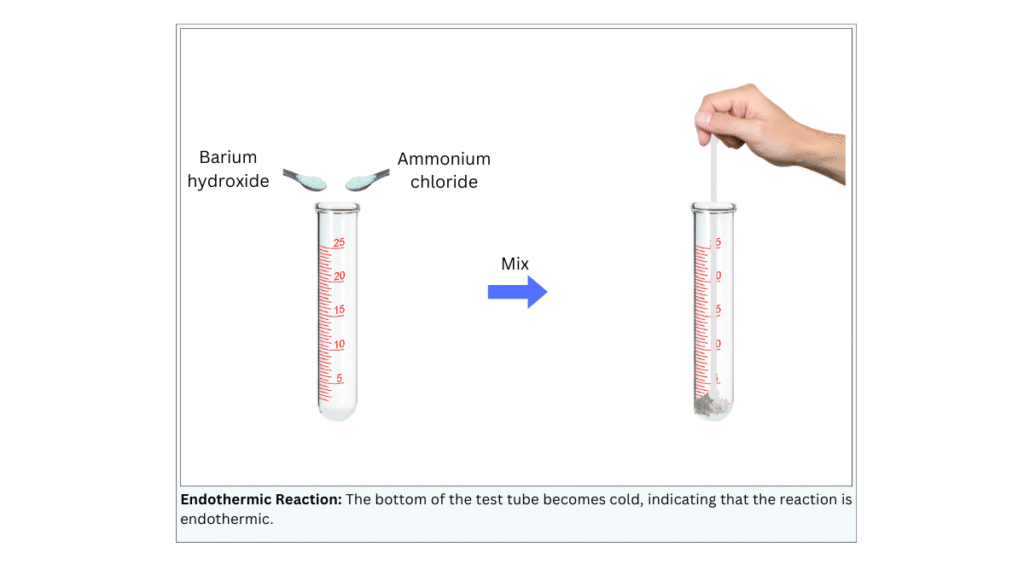
Conclusions:
- The reaction is an endothermic reaction because it absorbs heat from the surroundings.
- Barium chloride and ammonium hydroxide are formed. The double displacement reaction is shown below:
Ba(OH)2 + 2NH4Cl + heat → BaCl2 + 2NH4OH
“9. Carry out the following Activity (Page 10):
Take about 2 g barium hydroxide in a test tube. Add 1 g of ammonium chloride and mix with the help of a glass rod. Touch the bottom of the test tube with your palm. What do you feel? Is this an exothermic or endothermic reaction?” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 1.1
Solution to Activity 1.2
Solution to Activity 1.3
Solution to Activity 1.4
Solution to Activity 1.5
Solution to Activity 1.6
Solution to Activity 1.7
Solution to Activity 1.8
Solution to Activity
Solution to Activity 1.9
Solution to Activity 1.10
Solution to Activity 1.11


