6. Complete Activity 1.6 (Page 8).
- Take about 2 g lead nitrate powder in a boiling tube.
- Hold the boiling tube with a pair of tongs and heat it over a flame, as shown in Fig. 1.5.
- What do you observe? Note down the change, if any.
Answer:
Aim: To heat the boiling tube containing lead nitrate powder and conclude based on the observations.
Materials Required: 2 g lead nitrate powder, boiling tube, tongs, Bunsen burner.
Procedure:
(i) 2 g of lead nitrate powder is taken in a boiling tube.
(ii) Grasp the boiling tube with the pair of tongs and heat it over the flame of the burner. Note the observations.
Observations:
Brown fumes are emitted.
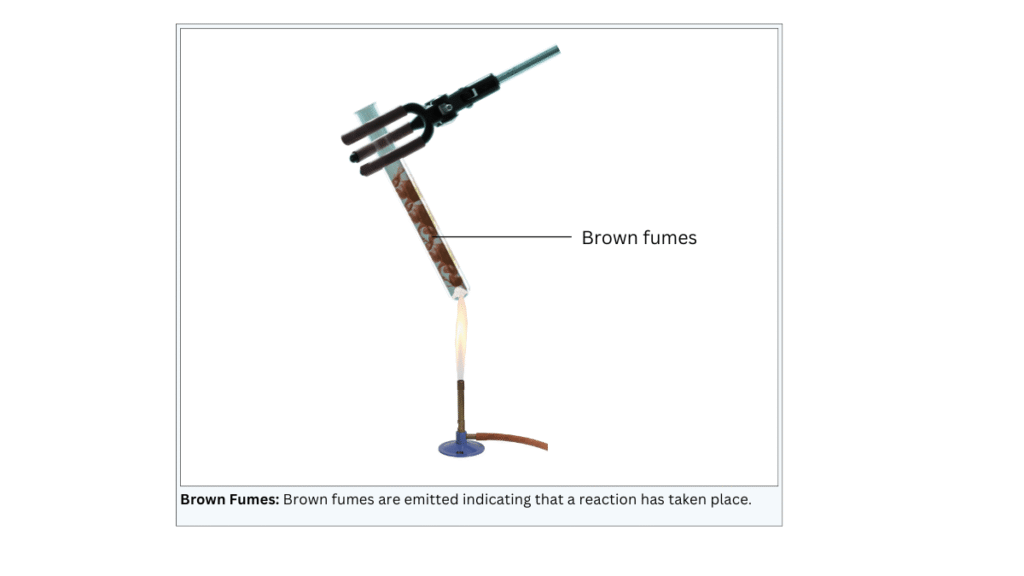
Conclusions:
- The brown fumes are of nitrogen dioxide.
- This indicates that a thermal decomposition reaction has taken place in which lead nitrate decomposes into lead oxide, nitrogen dioxide and oxygen. The reaction equation is shown below:
2Pb(NO3)2(s) + Heat —> 2PbO(s) + 4NO2(g) + O2(g)
“6. Complete Activity 1.6 (Page 8).
What do you observe? Note down the change, if any.
Take about 2 g lead nitrate powder in a boiling tube.
Hold the boiling tube with a pair of tongs and heat it over a flame, as shown in Fig. 1.5.” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 1.1
Solution to Activity 1.2
Solution to Activity 1.3
Solution to Activity 1.4
Solution to Activity 1.5
Solution to Activity 1.6
Solution to Activity 1.7
Solution to Activity 1.8
Solution to Activity
Solution to Activity 1.9
Solution to Activity 1.10
Solution to Activity 1.11


