11. Complete Activity 1.10 (Page 11).
- Take about 3 mL of sodium sulphate solution in a test tube.
- In another test tube, take about 3 mL of barium chloride solution.
- Mix the two solutions (Fig. 1.9).
- What do you observe?
Answer:
Aim: To observe what happens when sodium sulphate solution and barium chloride solution are mixed and conclude based on that.
Materials Required: 3 mL sodium sulphate solution, 3 mL barium chloride solution, two test tubes.
Procedure:
(i) In the first test tube, 3 mL sodium sulphate solution is taken.
(ii) In the second test tube, 3 mL of barium chloride solution is taken.
(iii) The two solutions are mixed together. The observations are noted.
Observations:
A white insoluble substance is formed.
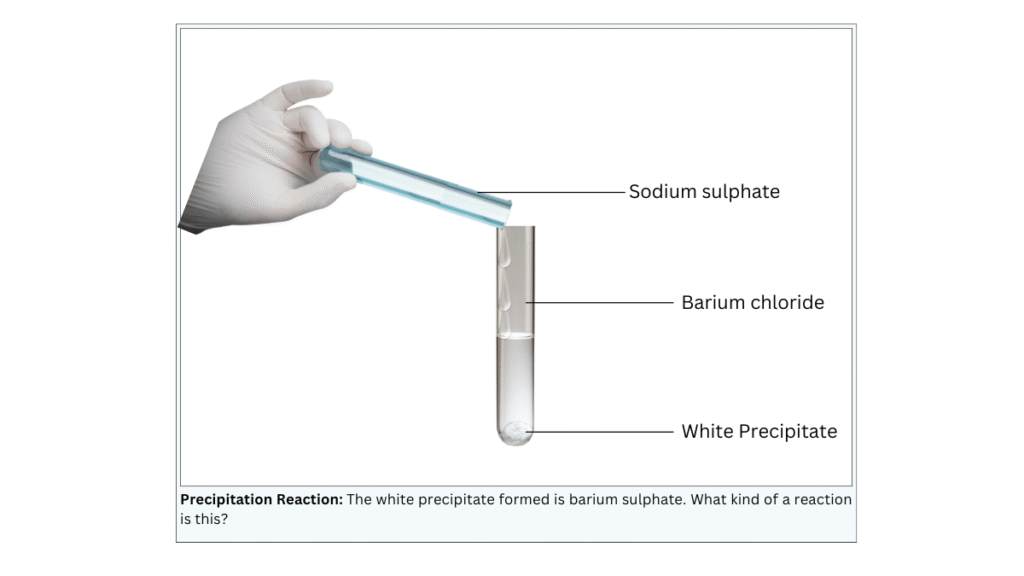
Conclusions:
- The insoluble substance is known as barium sulphate and is known as a precipitate.
- The reaction is a double displacement reaction and the equation is shown below:
- Na2SO4(aq) + BaCl2(aq) —> BaSO4(s) + 2NaCl(aq)
- Since this reaction produces a precipitate, it is known as a precipitation reaction.
“11. Complete Activity 1.10 (Page 11).
What do you observe?
Take about 3 mL of sodium sulphate solution in a test tube.
In another test tube, take about 3 mL of barium chloride solution.
Mix the two solutions (Fig. 1.9).” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 1.1
Solution to Activity 1.2
Solution to Activity 1.3
Solution to Activity 1.4
Solution to Activity 1.5
Solution to Activity 1.6
Solution to Activity 1.7
Solution to Activity 1.8
Solution to Activity
Solution to Activity 1.9
Solution to Activity 1.10
Solution to Activity 1.11


