2. Complete Activity 1.2 (Page 2).
- Take lead nitrate solution in a test tube.
- Add potassium iodide solution to this.
- What do you observe?
Answer:
Aim: To observe what happens when potassium iodide solution is added to lead nitrate solution and make necessary conclusions.
Materials Required: Lead nitrate,potassium iodide, test tube.
Procedure:
(i) Some lead nitrate solution is taken in a test tube.
(ii) Potassium iodide solution is added to it. Note the observations.
Observations:
A yellow precipitate is formed at the bottom of the test tube.
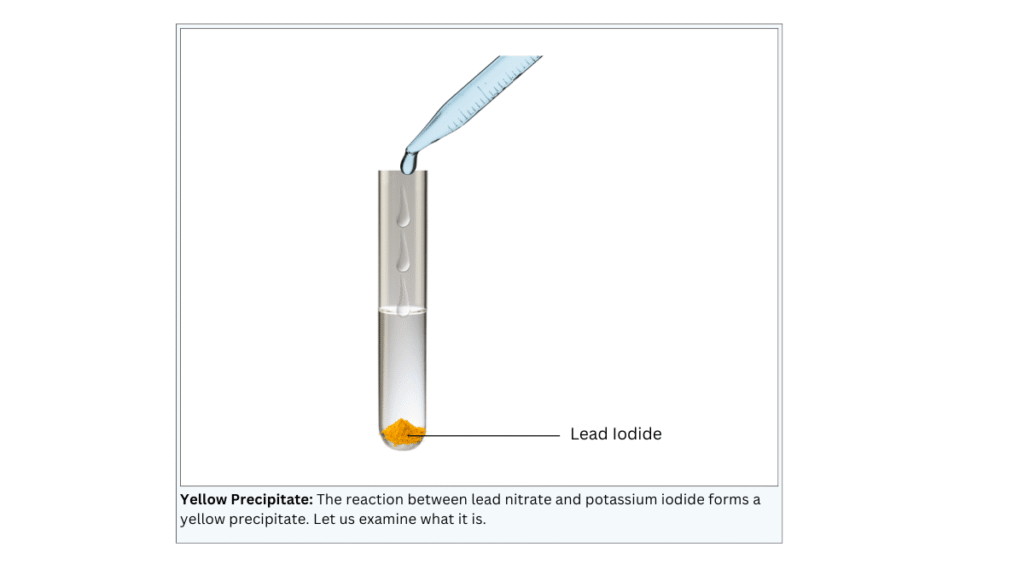
Conclusions:
- The yellow precipitate is potassium iodide.
- The formation of a new substance with new properties which indicates that a chemical reaction has taken place.
- The reaction is a double displacement reaction and the equation is shown below:
2KI(aq) + Pb(NO3)2(aq) —> 2KNO3(aq) + PbI2(s)
“Take lead nitrate solution in a test tube.
Add potassium iodide solution to this.
What do you observe?” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 1.1
Solution to Activity 1.2
Solution to Activity 1.3
Solution to Activity 1.4
Solution to Activity 1.5
Solution to Activity 1.6
Solution to Activity 1.7
Solution to Activity 1.8
Solution to Activity
Solution to Activity 1.9
Solution to Activity 1.10
Solution to Activity 1.11


