13. Complete Activity 3.13 (Page 48).
- Take samples of sodium chloride, potassium iodide, barium chloride or any other salt from the science laboratory.
- What is the physical state of these salts?
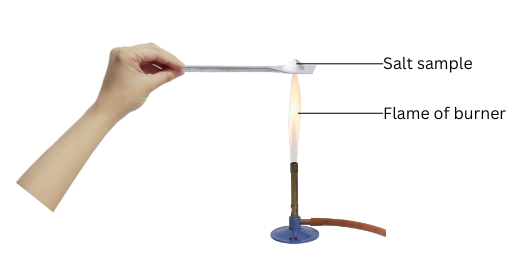
- Take a small amount of a sample on a metal spatula and heat directly on the flame (Fig. 3.7). Repeat with other samples.
- What did you observe? Did the samples impart any colour to the flame? Do these compounds melt?
- Try to dissolve the samples in water, petrol and kerosene. Are they soluble?
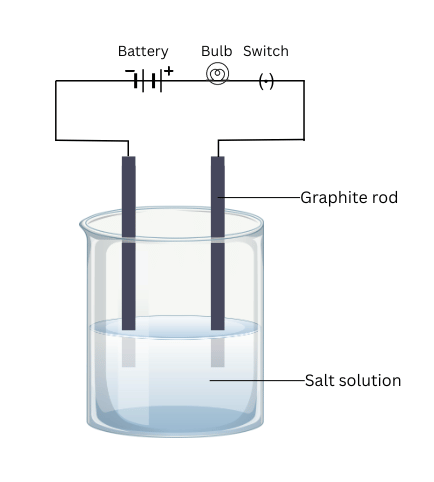
- Make a circuit as shown in Fig. 3.8 and insert the electrodes into a solution of one salt. What did you observe? Test the other salt samples too in this manner.
- What is your inference about the nature of these compounds?
Answer:
Aim:
Materials Required: Sodium chloride, potassium iodide, barium chloride, metal spatula, burner,water, petrol, kerosene, graphite electrodes, battery, bulb, switch, electrical wires.
Procedure:
(i) Take a small amount of each sample on the spatula and heat it directly on the flame of the burner. Note the change of colour of thee flame and whether these compound melt.
(ii) Try to dissolve each sample in water, petrol and kerosene and note whether they are soluble.
(iii) Design the circuit as shown and insert the electrodes into aqueous solutions of each salt. Note the observations.
Observations:
- Sodium chloride burns with a yellow flame, potassium iodide burns with a lilac flame and barium chloride burns with a green flame.
- None of the salts melt on being heated.
- Each of the salts are soluble in water, but insoluble in petrol and kerosene.
- Each of the salts conduct electricity when current is passed the salt solutions.
Conclusions:
- Ionic compounds are solids and are somewhat hard because of the strong force of attraction between the positive and negative ions.
- Ionic compounds have high melting and boiling points because a considerable amount of energy is required to break the strong inter-ionic forces of attraction.
- Ionic compounds are generally soluble in water and insoluble in solvents such as kerosene, petrol, etc.
- Ionic compounds conduct electricity in aqueous solution because ions are free to move in aqueous solution.
“Complete Activity 3.13 (Page 48).
- Take samples of sodium chloride, potassium iodide, barium chloride or any other salt from the science laboratory.
- What is the physical state of these salts?
- Take a small amount of a sample on a metal spatula and heat directly on the flame (Fig. 3.7). Repeat with other samples.
- What did you observe? Did the samples impart any colour to the flame? Do these compounds melt?
- Try to dissolve the samples in water, petrol and kerosene. Are they soluble?
- Make a circuit as shown in Fig. 3.8 and insert the electrodes into a solution of one salt. What did you observe? Test the other salt samples too in this manner.
- What is your inference about the nature of these compounds?” – Solved.
Related Links:
Solution to Activity 3.1
Solution to Activity 3.2
Solution to Activity 3.3
Solution to Activity 3.4
Solution to Activity 3.5
Solution to Activity 3.6
Solution to Activity 3.7
Solution to Activity 3.8
Solution to Activity 3.9
Solution to Activity 3.10
Solution to Activity 3.11
Solution to Activity 3.12
Solution to Activity 3.13
Solution to Activity 3.14


