
Answer:
Aim: To observe what happens when ammonium chloride is heated and infer based on the observations.
Materials Required: Ammonium chloride, china dish, funnel, cotton plug.
Procedure:
(i) Crush some of the ammonium chloride and place it in the china dish.
(ii) Place an inverted funnel over the china dish.
(iii) Close the opening of the stem of the funnel with a cotton plug.
(iv) Now heat the china dish using the burner and observe what happens.
Observations:
- The ammonium chloride vapourizes.
- Some ammonium chloride gets deposited on the walls of the funnel.
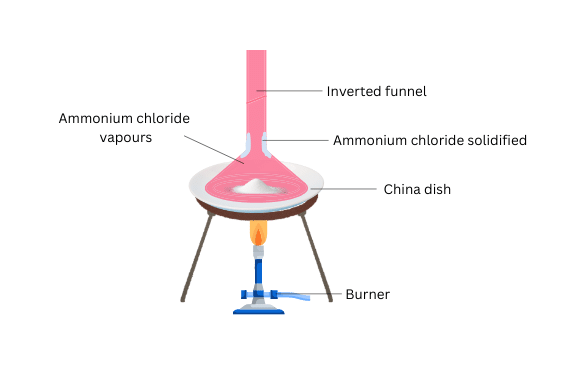
- Ammonium chloride undergoes sublimation and forms vapour directly from the solid state.
- The cotton plug prevents the vapour from escaping into the atmosphere. The vapour condenses on contact with the cooler walls of the funnel and gets deposited on the walls of the funnel directly as a solid. This process is the reverse of sublimation and is called deposition.
Related Links:
Solution to Group Activity
Solution to Activity 1.1
Solution to Activity 1.2
Solution to Activity 1.3
Solution to Activity 1.4
Solution to Activity 1.5
Solution to Activity 1.6
Solution to Activity 1.7
Solution to Activity 1.8
Solution to Activity 1.9
Solution to Activity 1.10
Solution to Activity 1.11
Solution to Activity 1.12
Solution to Activity 1.14
Solutions to Chapter 1 Matter in Our Surroundings


