
Answer:
Aim: To observe in which case the piston was easily pushed in and in which case it was not and infer from the observations.
Materials Required: Three 100 mL syringes, three rubber corks, water, pieces of chalk, vaseline.
Procedure:
(i) Close the nozzles of the three syringes with rubber corks.
(ii) Remove the pistons from the three syringes.
(iii) Leave the first syringe empty, fill water in the second and pieces of chalk in the third.
(iv) Apply some vaseline on the pistons for smooth movement and insert the piston back into the syringes.
(v) Try to compress the contents by pushing the piston in with force. Note the observations.
Observations:
- The piston was pushed in the most easily and farthest in case of the syringe filled with just air, followed by the syringe with water. The piston in the last syringe filled with pieces of chalk got pushed in the least amount.
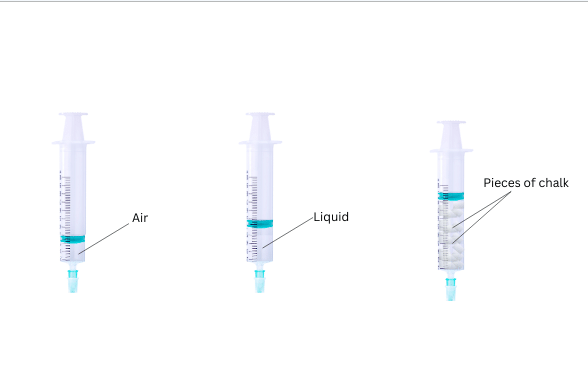
Conclusions: Gases are highly compressible due to larger spaces between particles, followed by liquids and then solids.
Related Links:
Solution to Group Activity
Solution to Activity 1.1
Solution to Activity 1.2
Solution to Activity 1.3
Solution to Activity 1.4
Solution to Activity 1.5
Solution to Activity 1.6
Solution to Activity 1.7
Solution to Activity 1.8
Solution to Activity 1.9
Solution to Activity 1.10
Solution to Activity 1.12
Solution to Activity 1.13
Solution to Activity 1.14
Solutions to Chapter 1 Matter in Our Surroundings


