2. Take three glass bottles with wide mouths. Label them A, B and C. Fill about half of bottle A with ordinary tap water. Fill bottle B with water which has been boiled for several minutes, to the same level as in A. In bottle C, take the same boiled water and of the amount as in other bottles. In each bottle put a few similar iron nails so that they are completely under water. Add a teaspoonful of cooking oil to the water in bottle C so it forms a film on its surface. Put the bottles away for a few days. Take out nails from each bottle and observe them. Explain your observations.
Answer:
The experiment can be described as follows:
Aim: To observe the rusting of iron nails under different conditions.
Materials Required: Threeglass bottles, tap water, boiled tap water, cooking oil.
Procedure:
(i) Take three glass bottles with wide open mouths and label them A, B and C.
(ii) Fill about half of bottle A with ordinary tap water.
(iii) Fill bottle B and bottle C with the same amount of water after boiling.
(iv) Submerge a few similar iron nails in bottles A, B and C.
(v) Add a teaspoon of cooking oil to bottle C which forms a yellow film on the surface. (vi) Put the bottles away for a few days.
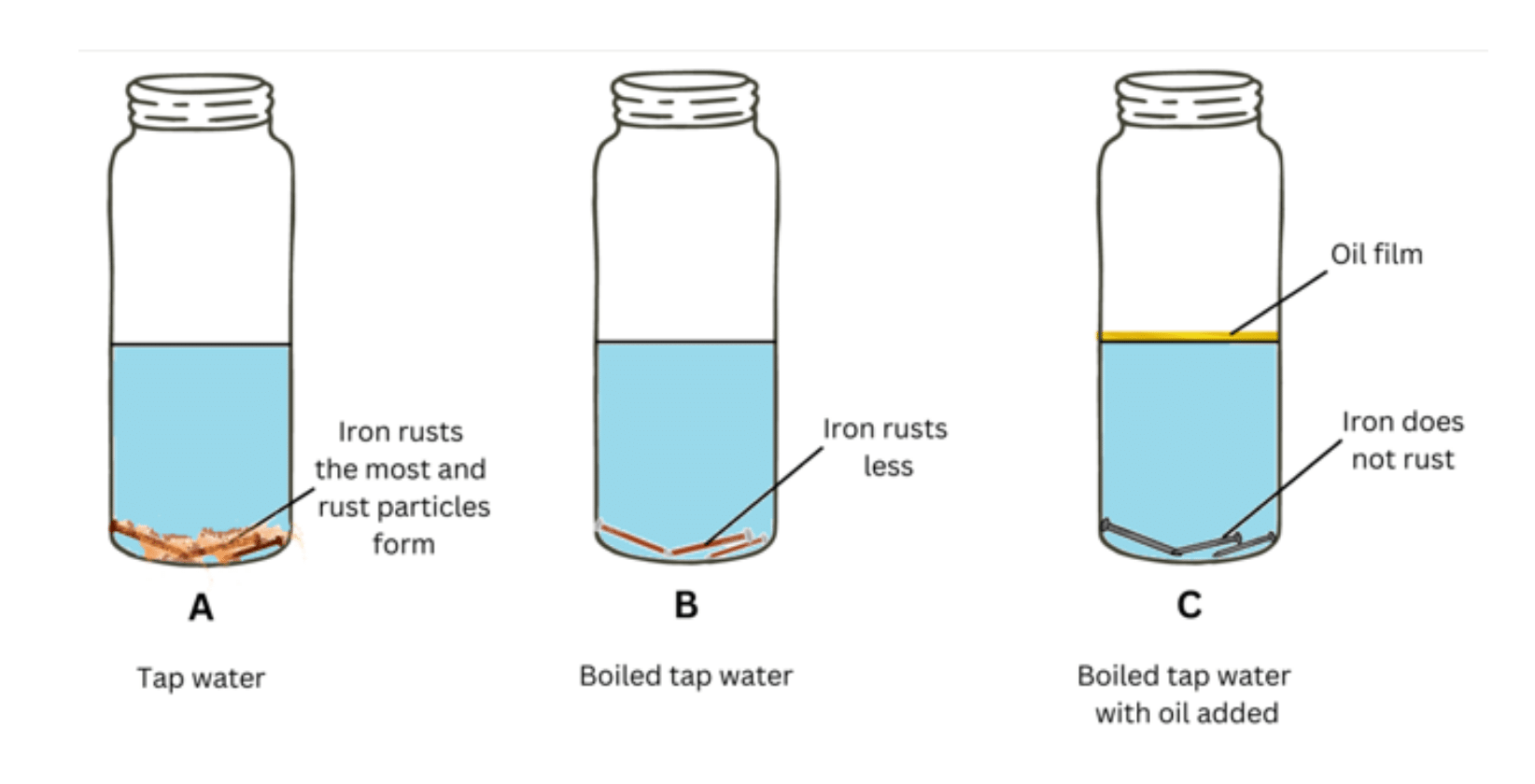
Observations: As shown in the figure: the nails in Bottle A get discoloured and develop rust and rust particles form, the nails in Bottle B develop partial rust and discolouration and nails in Bottle C do not develop rust.
Conclusions: For rust to form we need oxygen and water.
Bottle A contains pure tap water which has dissolved oxygen in it. This creates an ideal condition for rust to form.
Bottle B contains boiled tap water which contains much less amount of oxygen because it is removed (oxygen bubbles moving upward) during boiling. So, in Bottle B although same amount of water is present, the amount of dissolved oxygen is less and so partial rusting occurs.
Lastly, in Bottle C same amount of water is present, but the water is boiled due to which oxygen is removed and the oil film at the surface prevents the oxygen from the atmosphere from entering the water. Thus, the dissolved oxygen content in water is very less and the iron does not rust at all.
“Take three glass bottles with wide mouths. Label them A, B and C. Fill about half of bottle A with ordinary tap water. Fill bottle B with water which has been boiled for several minutes, to the same level as in A. In bottle C, take the same boiled water and of the amount as in other bottles. In each bottle put a few similar iron nails so that they are completely under water. Add a teaspoonful of cooking oil to the water in bottle C so it forms a film on its surface. Put the bottles away for a few days. Take out nails from each bottle and observe them. Explain your observations.” – Solved.
Related Links:
Solution to Extended Learning – Activities and Projects Question 1
Solution to Extended Learning – Activities and Projects Question 3
Solution to Extended Learning – Activities and Projects Question 4
Solution to Activity 5.1
Solution to Activity 5.2
Solution to Activity 5.3
Solution to Activity 5.4
Solution to Activity 5.5
Solution to Activity 5.6
Solution to Activity 5.7
Solution to Activity 5.8
Solutions to Chapter 5 Physical and Chemical Changes


