10. Complete Activity 1.9 (Page 10).
- Take three iron nails and clean them by rubbing with sand paper.
- Take two test tubes marked as (A) and (B). In each test tube, take about 10 mL copper sulphate solution.
- Tie two iron nails with a thread and immerse them carefully in the copper sulphate solution in test tube B for about 20 minutes [Fig. 1.8 (a)]. Keep one iron nail aside for comparison.
- After 20 minutes, take out the iron nails from the copper sulphate solution.
- Compare the intensity of the blue colour of copper sulphate solutions in test tubes (A) and (B) [Fig. 1.8 (b)].
- Also, compare the colour of the iron nails dipped in the copper sulphate solution with the one kept aside [Fig. 1.8 (b)].
Answer:
Aim: To compare the colour of the copper sulphate solutions with each other and also the colour of the two iron nails with each other and conclude based on that.
Materials Required: Three iron nails, sand paper, two test tubes, copper sulphate solution, thread,
Procedure:
(i) The iron nails are cleaned by rubbing with sand paper.
(ii) In test tube named A 10 mL of copper sulphate solution is taken.
(iii) In test tube named B 10 mL of copper sulphate solution is taken and two iron nails are immersed in the solution with a thread.
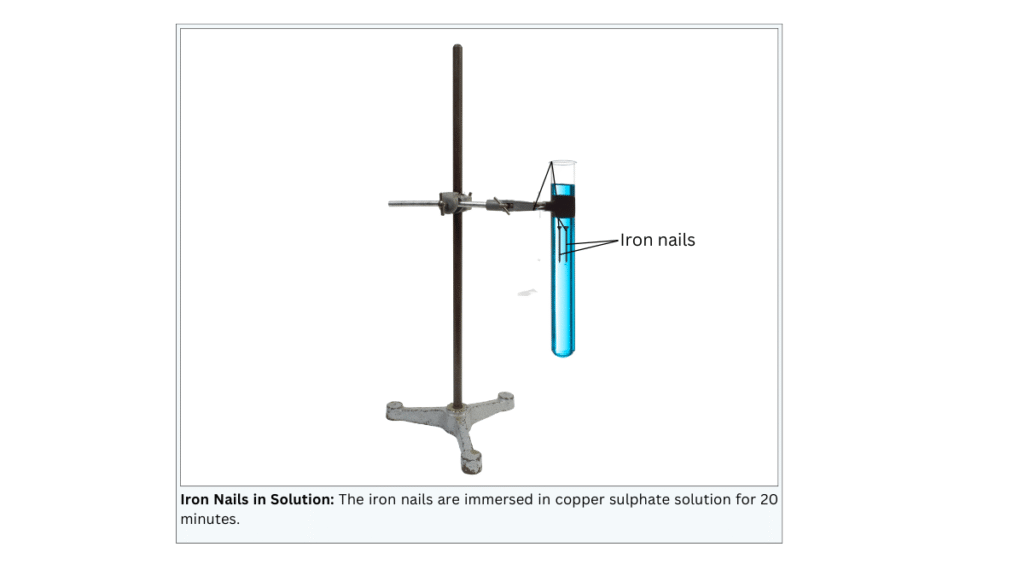
(iv) The third nail is kept aside for comparison.
(v) After 20 minutes the two iron nails are removed from the copper sulphate solution.
(vi) The intensity of the blue colour of the copper sulphate solutions in test tubes (A) and (B) are compared.
(vii) The colour of the iron nails immersed in the copper sulphate solution are compared with the iron nail that was kept aside.
Observations:
- The copper sulphate solution in test tube A remained blue, while the copper sulphate solution in test tube B faded.
- The colour of the iron nails immersed in the copper sulphate solution became brown, while the iron nail that was kept aside retained its normal colour.
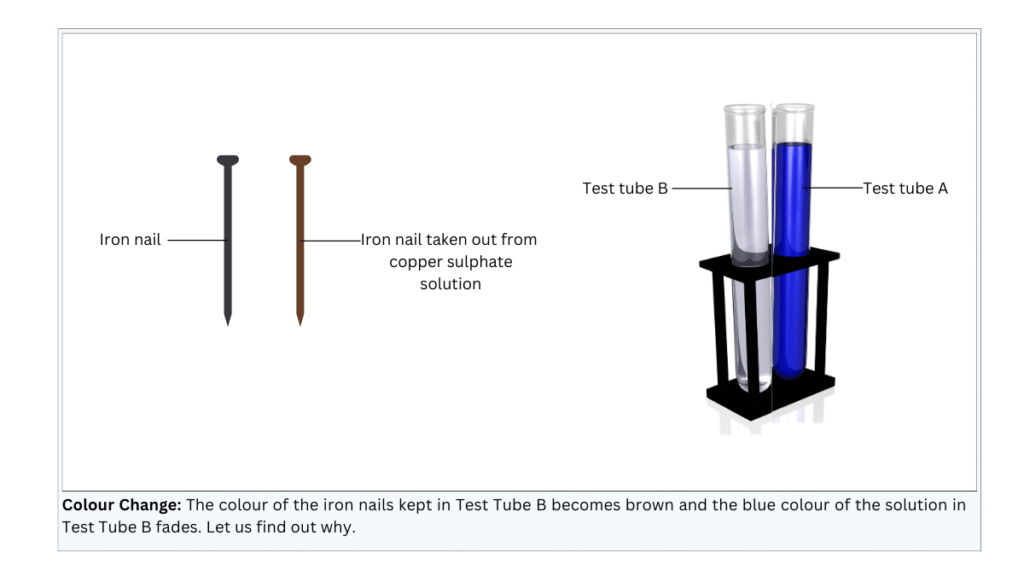
Conclusions:
- A displacement reaction occurs in which iron being more reactive than copper, displaces copper from copper sulphate. This copper gets deposited on the iron nail, resulting in the brown colour.
- Iron sulphate is formed as a result of which copper sulphate solution fades in colour.
- The displacement reaction equation is shown below:
Fe(s) + CuSO4(aq) —> FeSO4(aq) + Cu(s)
“10. Complete Activity 1.9 (Page 10).
Take three iron nails and clean them by rubbing with sand paper.
Take two test tubes marked as (A) and (B). In each test tube, take about 10 mL copper sulphate solution.
Tie two iron nails with a thread and immerse them carefully in the copper sulphate solution in test tube B for about 20 minutes [Fig. 1.8 (a)]. Keep one iron nail aside for comparison.
After 20 minutes, take out the iron nails from the copper sulphate solution.
Compare the intensity of the blue colour of copper sulphate solutions in test tubes (A) and (B) [Fig. 1.8 (b)].” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 1.1
Solution to Activity 1.2
Solution to Activity 1.3
Solution to Activity 1.4
Solution to Activity 1.5
Solution to Activity 1.6
Solution to Activity 1.7
Solution to Activity 1.8
Solution to Activity
Solution to Activity 1.9
Solution to Activity 1.10
Solution to Activity 1.11


