14. Complete Activity 3.14 (Page 53).
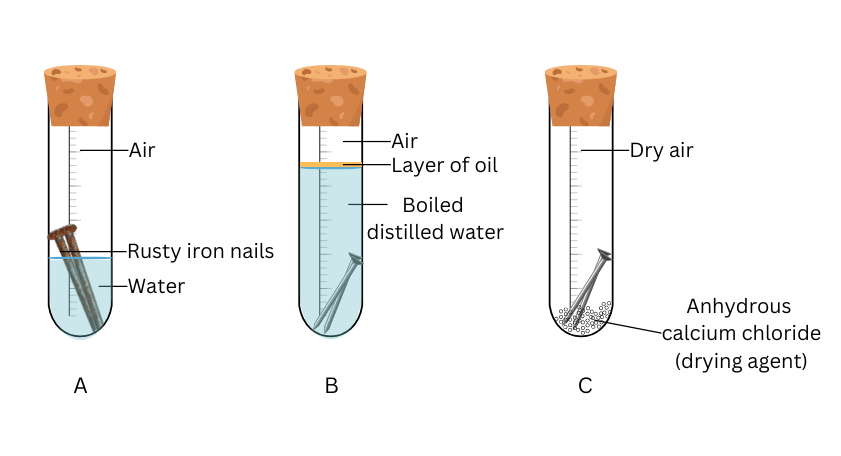
- Take three test tubes and place clean iron nails in each of them.
- Label these test tubes A, B and C. Pour some water in test tube A and cork it.
- Pour boiled distilled water in test tube B, add about 1 mL of oil and cork it. The oil will float on water and prevent the air from dissolving in the water.
- Put some anhydrous calcium chloride in test tube C and cork it. Anhydrous calcium chloride will absorb the moisture, if any, from the air. Leave these test tubes for a few days and then observe (Fig. 3.13).
Answer:
Aim: To observe what happens in the test tubes A, B and C after a few days and conclude based on the observations.
Materials Required: Three test tubes, three corks, 6 clean iron nails, boiled distilled water, oil, anhydrous calcium chloride.
Procedure:
(i) Place two clean iron nails in each test tube and label the test tubes as A, B and C.
(ii) Pour normal water in test tube A and cork it.
(iii) Pourboiled distilled water in test tube B, add about 1 mL of oil and cork it.
(iv) Take some anhydrous calcium chloride in test tube C and cork it.
(v) Note what happens to the iron nails in the three test tubes after a few days.
Observations:
- The iron nails in test tube A will rust.
- The iron nails in test tubes B and C will not rust.
Conclusions:
- In test tube A, the nails are exposed to both air and water, which are ideal conditions for rusting.
- In test tube B, the layer of oil acts as a barrier against air dissolving in water. Also, the boiled distilled water does not contain any oxygen due to removal by boiling. Therefore, the nails are exposed to only water and do not rust.
- In test tube C there is not water. In addition, the air is dried of moisture by anhydrous calcium chloride. Hence, the iron nails do not rust.
- We conclude that both oxygen and moisture are necessary for rusting to occur.
“14. Complete Activity 3.14 (Page 53).
- Take three test tubes and place clean iron nails in each of them.
- Label these test tubes A, B and C. Pour some water in test tube A and cork it.
- Pour boiled distilled water in test tube B, add about 1 mL of oil and cork it. The oil will float on water and prevent the air from dissolving in the water.
- Put some anhydrous calcium chloride in test tube C and cork it. Anhydrous calcium chloride will absorb the moisture, if any, from the air. Leave these test tubes for a few days and then observe (Fig. 3.13).” – Solved.
Related Links:
Solution to Activity 3.1
Solution to Activity 3.2
Solution to Activity 3.3
Solution to Activity 3.4
Solution to Activity 3.5
Solution to Activity 3.6
Solution to Activity 3.7
Solution to Activity 3.8
Solution to Activity 3.9
Solution to Activity 3.10
Solution to Activity 3.11
Solution to Activity 3.12
Solution to Activity 3.13
Solution to Activity 3.14


