5. Complete Activity 2.5 (Page 20).
- Take two test tubes, label them as A and B.
- Take about 0.5 g of sodium carbonate (Na2CO3) in test tube A and about 0.5 g of sodium hydrogencarbonate (NaHCO3) in test tube B.
- Add about 2 mL of dilute HCl to both the test tubes.
- What do you observe?
- Pass the gas produced in each case through lime water (calcium hydroxide solution) as shown in Fig. 2.2 and record your observations.
Answer:
Aim: To react sodium carbonate and sodium hydrogencarbonate with HCl and conclude based on the observations.
Materials Required: Two test tubes, two corks, two laboratory stands, two thistle funnels, two delivery tubes, sodium carbonate, sodium hydrogencarbonate, hydrogen chloride, calcium hydroxide solution.
Procedure:
(i) Mark the test tubes as A and B and attached each to a laboratory stand.
(ii) Take about 0.5 g of sodium carbonate (Na2CO3) in test tube A and about 0.5 g of sodium hydrogencarbonate (NaHCO3) in test tube B.
(iii) Add about 2 mL of dilute HCl to both the test tubes and close the mouths of the test tubes with the corks. Before that make sure you insert the delivery tube through the cork as shown in the figure. Note the observations.
(iv) Now submerge the other end of the delivery tube into a solution of lime water. Nore the observations.
Observations:
- After adding HCl to the test tubes, bubbling is observed in both cases.
- The lime water turns milky in both cases.
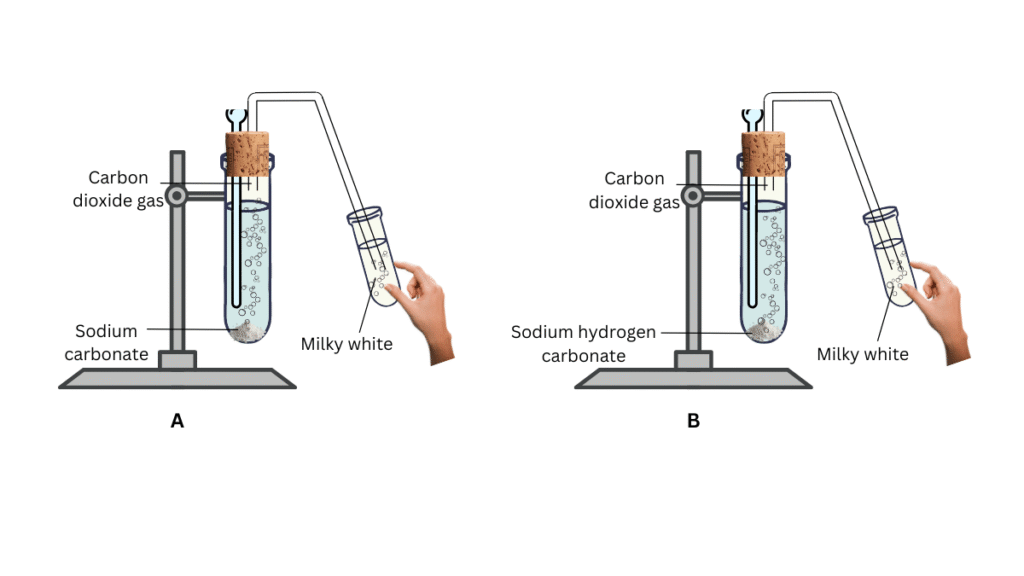
Conclusions:
- Since bubbling occurs and lime water turns milky, we can say that carbon dioxide gas is evolved in both cases.
- The reactions occurring in the test tubes are as follows:
Test tube A: Na2CO3(s) + 2HCl(aq) —> 2NaCl(aq) + H2O(l) + CO2(g)
Test tube B: NaHCO3(s) + 2HCl(aq) —> NaCl(aq) + H2O(l) + CO2(g)
- On passing carbon dioxide gas through lime water, a white precipitate calcium carbonate is formed which gives the milky white appearance.
Ca(OH)2(aq) + CO2(g) —> CaCO3(s) + H2O(l) - We can generalise these results and conclude that all metal carbonates and hydrogencarbonates react with acids to give a corresponding salt, carbon dioxide and water.
“5. Complete Activity 2.5 (Page 20).
Pass the gas produced in each case through lime water (calcium hydroxide solution) as shown in Fig. 2.2 and record your observations.
Take two test tubes, label them as A and B.
Take about 0.5 g of sodium carbonate (Na2CO3) in test tube A and about 0.5 g of sodium hydrogencarbonate (NaHCO3) in test tube B.
Add about 2 mL of dilute HCl to both the test tubes.
What do you observe?” – Solved.
Related Links:
Solution to Group Activity
Solution to Activity 2.1
Solution to Activity 2.2
Solution to Activity 2.3
Solution to Activity 2.4
Solution to Activity 2.5
Solution to Activity 2.6
Solution to Activity 2.7
Solution to Activity 2.8
Solution to Activity 2.9
Solution to Activity 2.10
Solution to Activity 2.11
Solution to Activity 2.12
Solution to Activity 2.13
Solution to Activity 2.14
Solution to Activity 2.15


